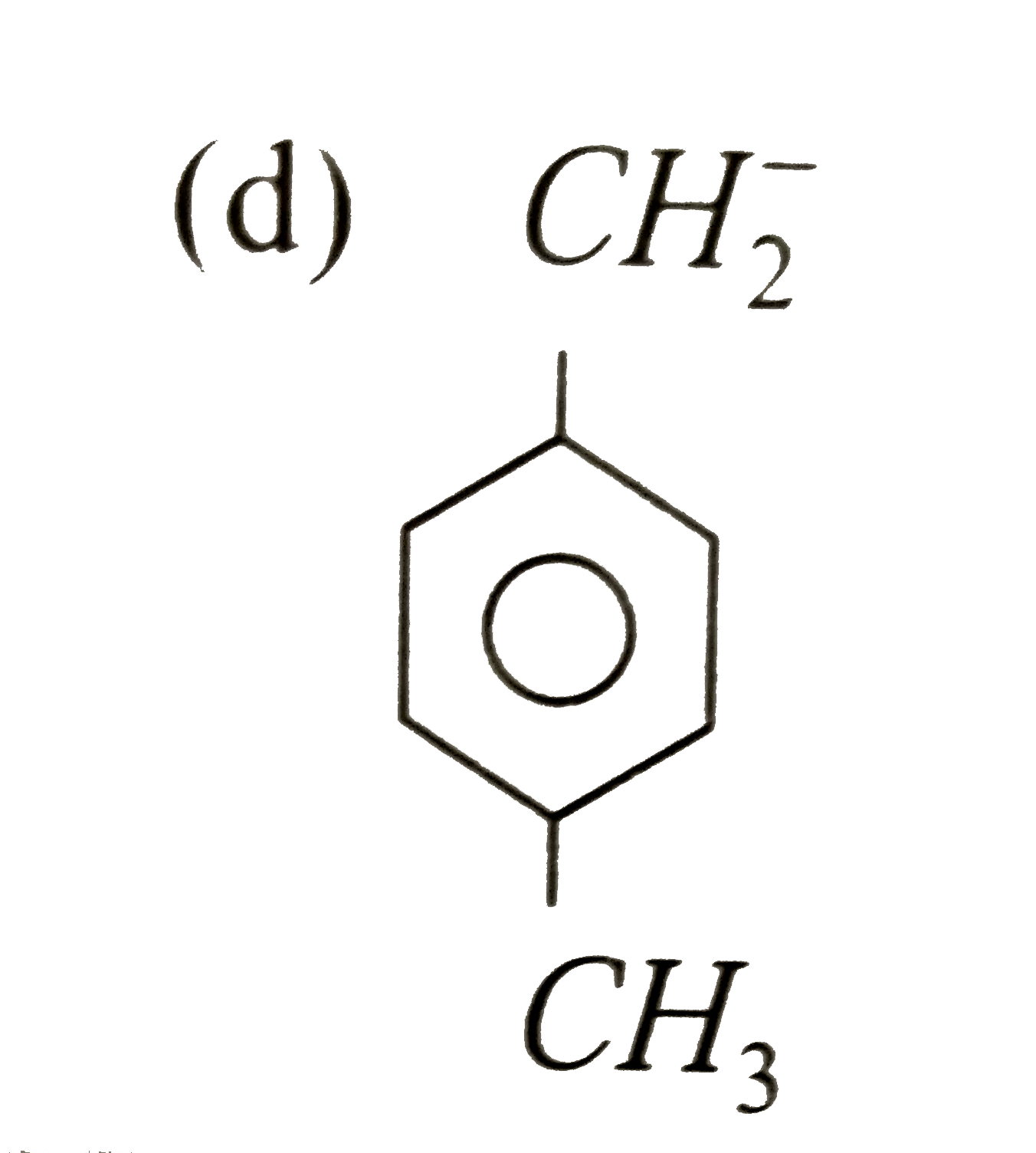Methyl carbanion is................and...............with ammonia.
The correct Answer is:isoelectronic, isostructural
To answer the question "Methyl carbanion is................and...............with ammonia," we need to analyze the properties of methyl carbanion (CH3⁻) and ammonia (NH3).
1. Identify the Structure of Methyl Carbanion (CH3⁻):
- Methyl carbanion has the formula CH3⁻. It consists of one carbon atom bonded to three hydrogen atoms and carries a negative charge.
- The carbon atom has 4 valence electrons, and with the addition of one extra electron (due to the negative charge), it has a total of 5 electrons.
2. Identify the Structure of Ammonia (NH3):
- Ammonia has the formula NH3. It consists of one nitrogen atom bonded to three hydrogen atoms.
- Nitrogen has 5 valence electrons, and with 3 hydrogen atoms (each contributing 1 electron), it has a total of 8 electrons.
3. Compare the Electron Count:
- Methyl carbanion (CH3⁻) has 10 electrons (4 from carbon and 6 from hydrogen).
- Ammonia (NH3) has 10 electrons (5 from nitrogen and 5 from hydrogen).
- Since both species have the same number of electrons, they are iso-electronic.
4. Compare the Geometry:
- The geometry of methyl carbanion is tetrahedral due to the four electron pairs (three bonding pairs and one lone pair).
- Ammonia also has a tetrahedral shape but is often described as having a trigonal pyramidal geometry due to the presence of a lone pair on nitrogen.
- Therefore, methyl carbanion and ammonia are iso-structural as well.
5. Final Answer:
- Methyl carbanion is iso-electronic and iso-structural with ammonia.
- Methyl carbanion has the formula CH3⁻. It consists of one carbon atom bonded to three hydrogen atoms and carries a negative charge.
- The carbon atom has 4 valence electrons, and with the addition of one extra electron (due to the negative charge), it has a total of 5 electrons.
- Ammonia has the formula NH3. It consists of one nitrogen atom bonded to three hydrogen atoms.
- Nitrogen has 5 valence electrons, and with 3 hydrogen atoms (each contributing 1 electron), it has a total of 8 electrons.
- Methyl carbanion (CH3⁻) has 10 electrons (4 from carbon and 6 from hydrogen).
- Ammonia (NH3) has 10 electrons (5 from nitrogen and 5 from hydrogen).
- Since both species have the same number of electrons, they are iso-electronic.
- The geometry of methyl carbanion is tetrahedral due to the four electron pairs (three bonding pairs and one lone pair).
- Ammonia also has a tetrahedral shape but is often described as having a trigonal pyramidal geometry due to the presence of a lone pair on nitrogen.
- Therefore, methyl carbanion and ammonia are iso-structural as well.
- Methyl carbanion is iso-electronic and iso-structural with ammonia.
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Conceptual Questions (I. Hybridization Classification and Nomenclature)
Explore 4 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Conceptual Questions (II. Isomerism)
Explore 9 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Test Your Grip (I. Multiple Choice Questions)
Explore 1 Video
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)
Explore 10 Videos
Similar Questions
Knowledge Check
Most stable carbanion is
ACH−3BCH3CH−2C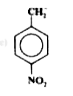 D
D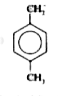
Most stable carbanion is :-
AHC≡C−BC6H−5C(CH3)3C−CH−2D(CH3)2C=CH−Most stable carbanion is
ACH−3BCH3CH−2C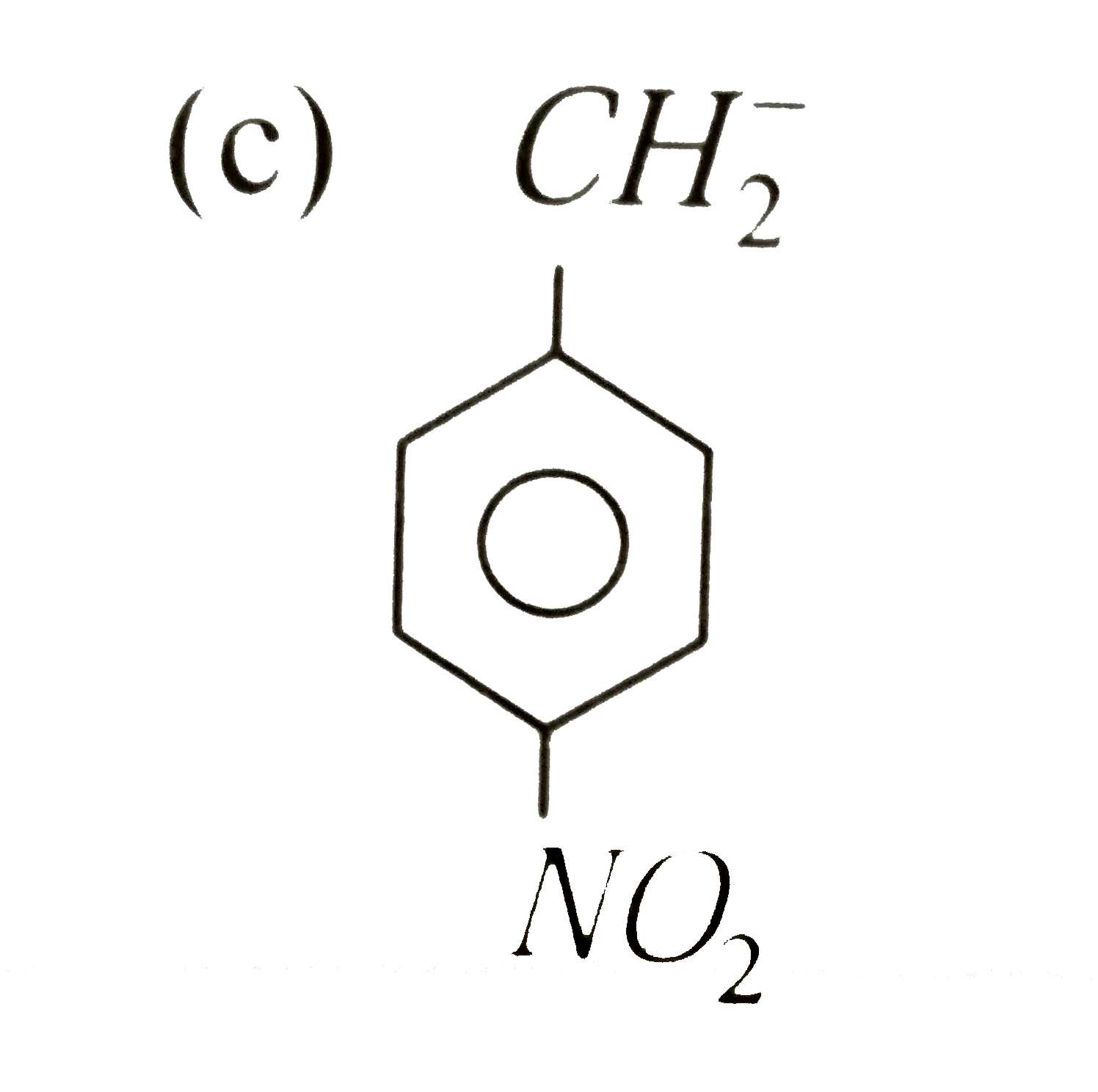 D
D