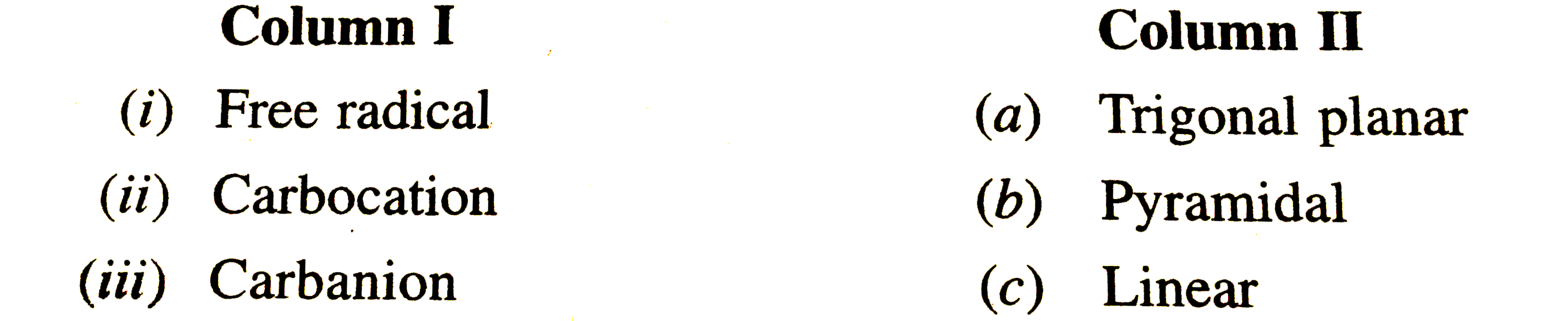
Match the intermediates given in Column I with their probable structure in Column II.
Video Solution
Text Solution
(i)→(a),(b),(ii)→(a),(iii)→(a),(b).
Note : Please note that simple free radicals are trigonal planar but bridge head free radicals are pyramidal. Similarly, simple carbanions are pyramidal while those in which the carbon atom carrying the negative charge is adjacent to a double bond or a benzene ring are trigonal planar.
|
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Assertion and Reason Type Questions)
Explore 6 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Long Answer Type Questions)
Explore 6 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Short Answer Questions)
Explore 19 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)
Explore 10 Videos
Similar Questions
Knowledge Check
Match the polymers given in Column I with their commercial names given in Column II.
 AA-4,B-3,C-2,D-1,E-5BA-2,B-3,C-1,D-5,E-4CA-2,B-1,C-3,D-4,E-5DA-2,B-1,C-3,D-5,E-4
AA-4,B-3,C-2,D-1,E-5BA-2,B-3,C-1,D-5,E-4CA-2,B-1,C-3,D-4,E-5DA-2,B-1,C-3,D-5,E-4
Match the statements given in Column I with the oxidation states given in Column II.

View SolutionMatch the reactions given in Column I with the suitable reagents given in Column II.

View SolutionMatch the reactions given in Column I with the types of reactions given in Column II.
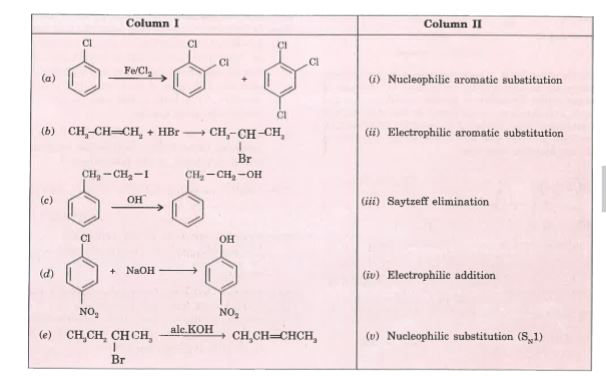
View SolutionMatch the reactions given in Column I with the types of reactions given in Column II.

View SolutionMatch the reactions given in Column I with the types of reactions given in Column II.

View SolutionMatch the polymers given in Column I with their commercial names given in Column II.

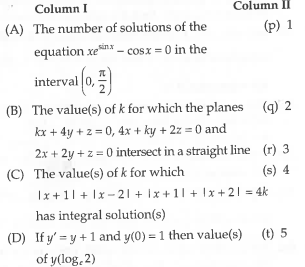
View SolutionMatch the statements/expressions given in Column I with the values given in Column II.
View Solution

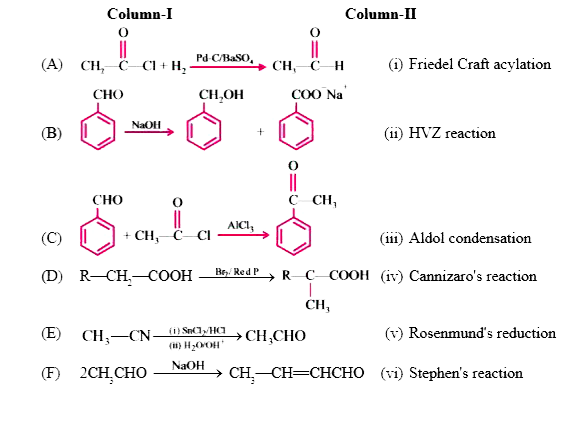 .
. 