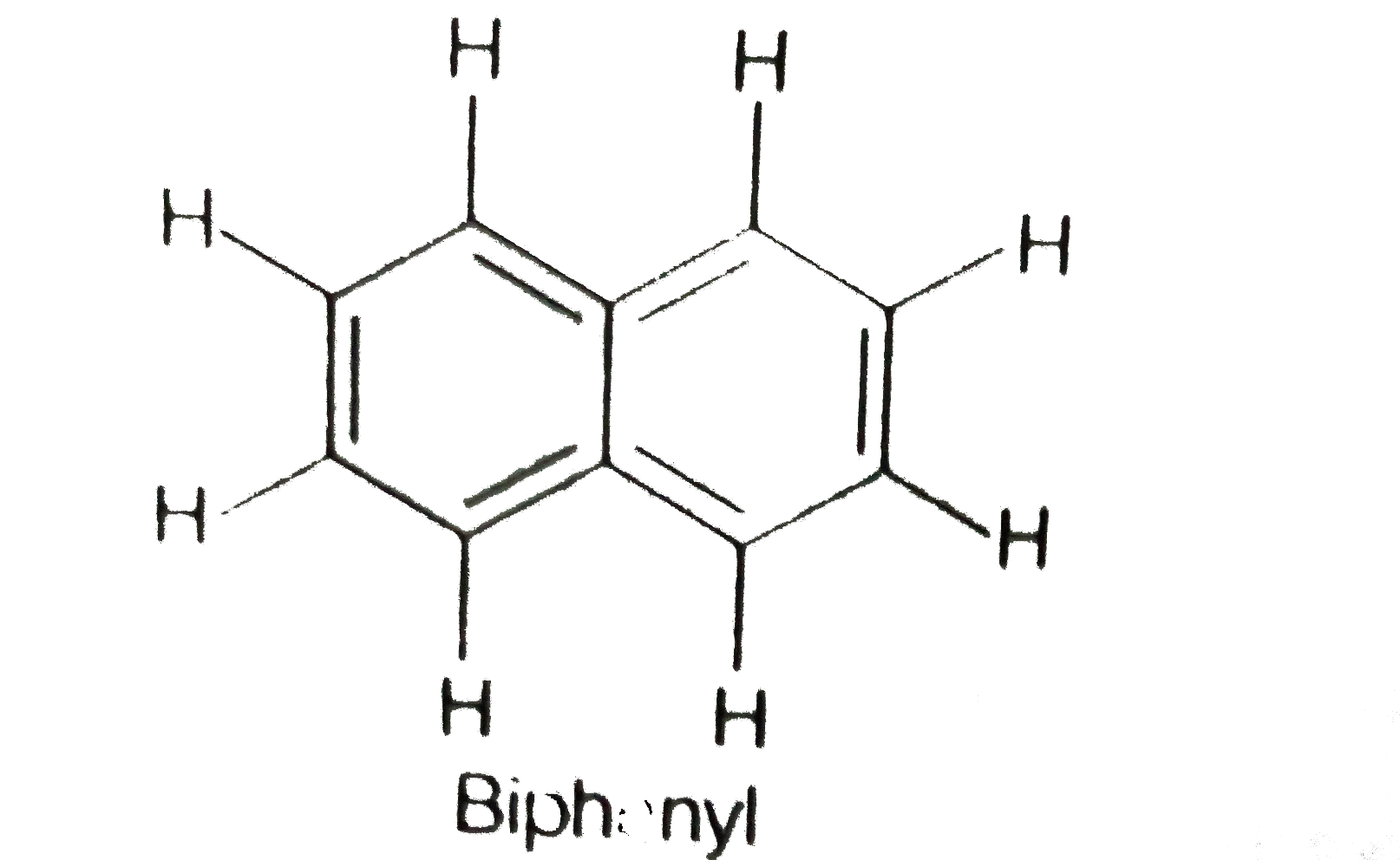
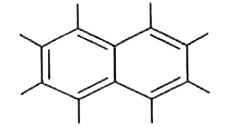
The total number of π-bond electrons in the following structure is
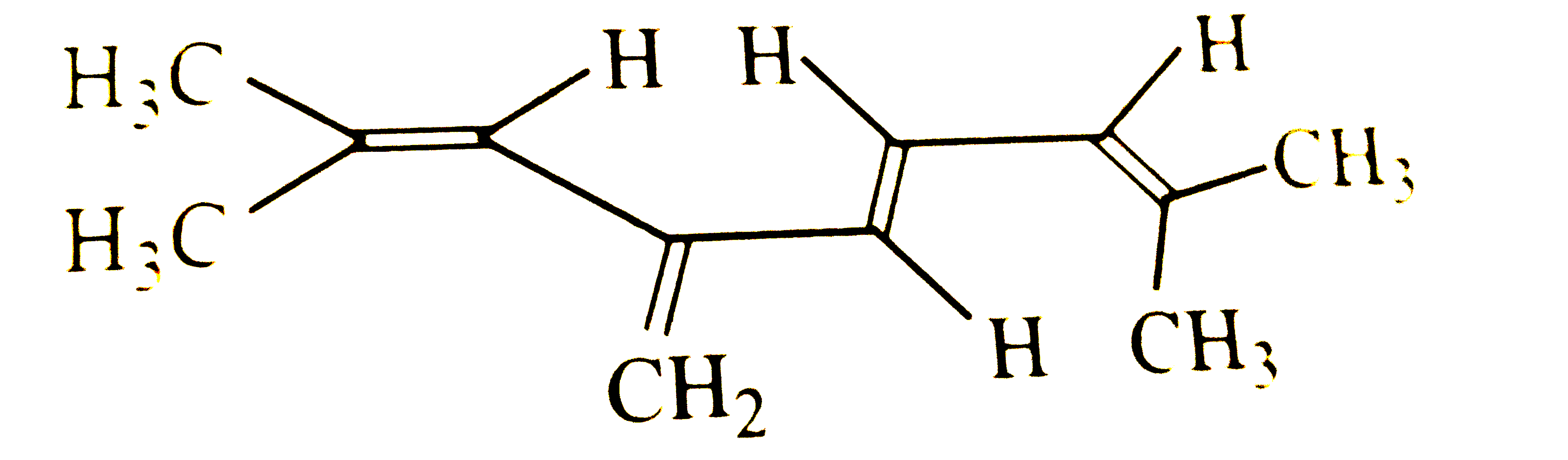
12
16
4
8
The correct Answer is:D
No. of π-bonds =4
∴ No. of π electrons =4×2=8
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Competition Focus (Jee (Main and Advanced)/Medical Entrance) II. Multiple Choice Questions (with one or more than one correct answers)
8 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Competition Focus (Jee (Main and Advanced)/Medical Entrance) III. Multiple Choice Questions (Comprehension)
12 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Analytical Questions and Problems with Answers/Solutions (Problems)
4 Videos
- HYDROGEN BOOK - PRADEEPCHAPTER - HYDROGEN EXERCISE - COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
15 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)BOOK - PRADEEPCHAPTER - P-BLOCK ELEMENTS (NITROGEN FAMILY)EXERCISE - Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)
10 Videos
Similar Questions
Which of the following compounds contain only sp3 hybridized carbon ?
View SolutionHere paragraphs are given. Based upon the paragraphs some multiple choice questions are given. Each question has 4 choices A, B, C and D. Out of which one is correct. Choose the correct option
Paragraph/comprehension
In the detection of elements by Lassaigne’s test, the covalent compounds are converted into ionic compounds on fusion with sodium metal. The nitrogen, sulphur and halides present in the organic compounds are test with their usual tests. In this test blue or green colour is obtained when only nitrogen is present whereas red colour is obtained when both nitrogen and sulphur are present. The Lassaigne’s extract is boiled with HNO3 so as to decompose Na2 and NaCN if present. Phosphorus is detected in the organic compound by fusing it with sodium peroxide. When sodium phosphate formed is detected with cone. HNO3 and ammonium molybdate.
An organic compound containing N, S and O as extra elements is fused with sodium metal and then extracted with water. The species which is not present in the solution of the extract is
View Solution1.216 gm of an organic compound was reacted under Kjeldahl's method and the ammonia evolved was absorbed in 100 ml NH2SO4. The remaining acid solution was made up to 500 ml by the addition of water. Twenty millilitres of the dilute solution required 32 ml N10 caustic soda solution for complete neutralisation. Calculate the percentage of nitrogen in the compound.
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View SolutionStatement -1 : Although NaH2PO2 contains two H-atoms, it is not an acid salt.
Satement -2 : It contains two ionisable hydrogens.
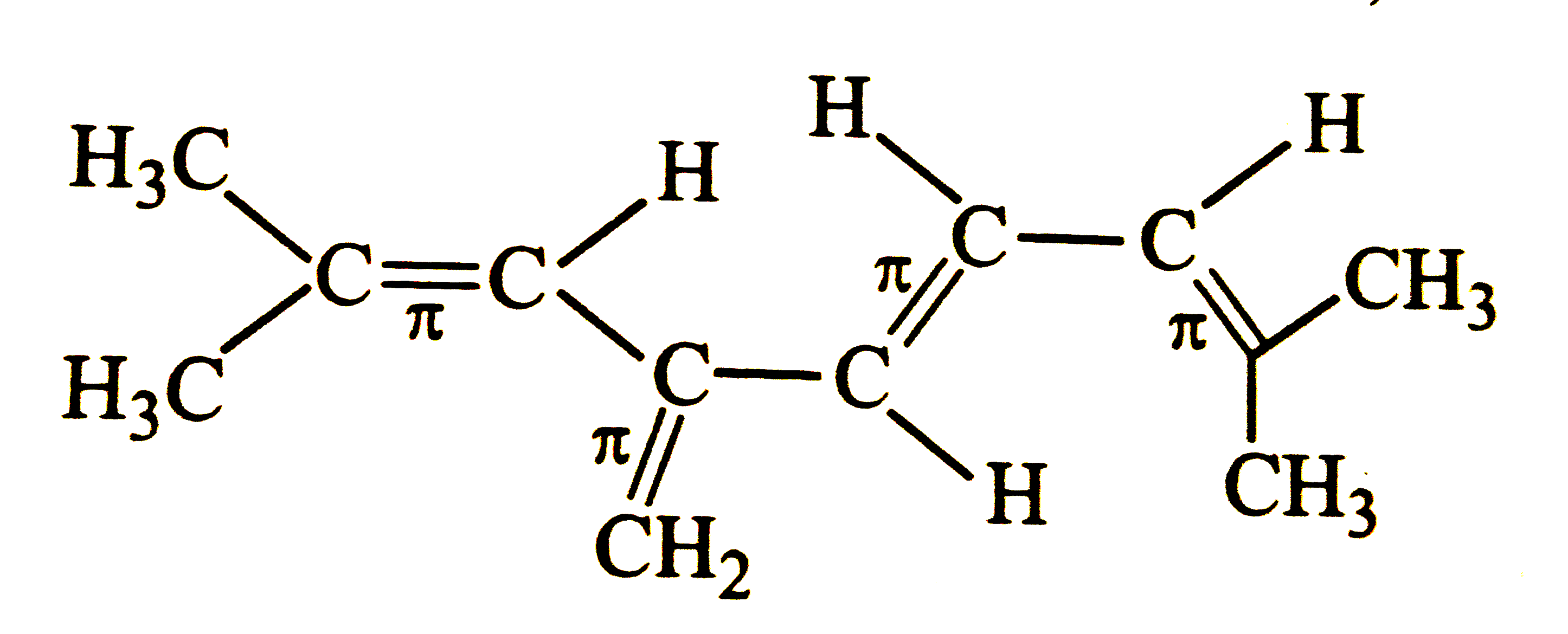
View SolutionThe total number of pi bond electrons in the following structure is
View SolutionThe total number of p− bond electrons in the following structure is
View SolutionThe total number of π-bond electrons in the following structure is
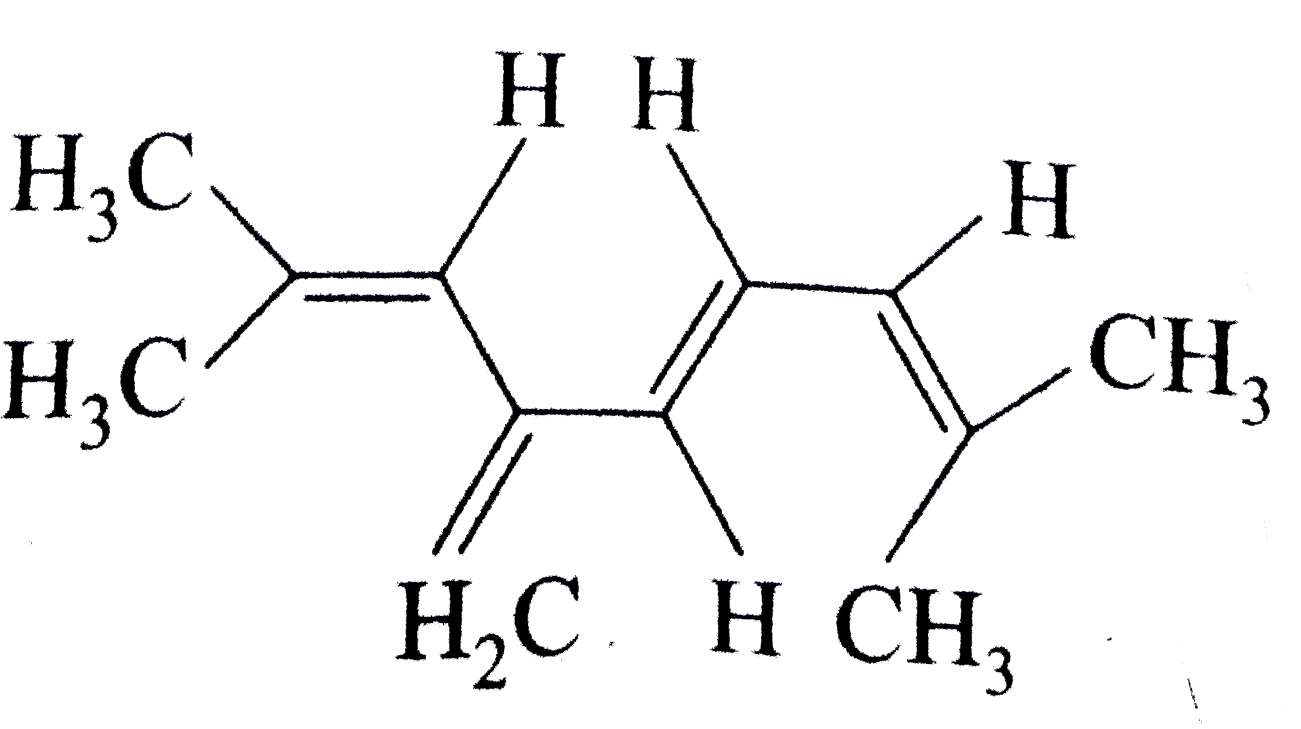
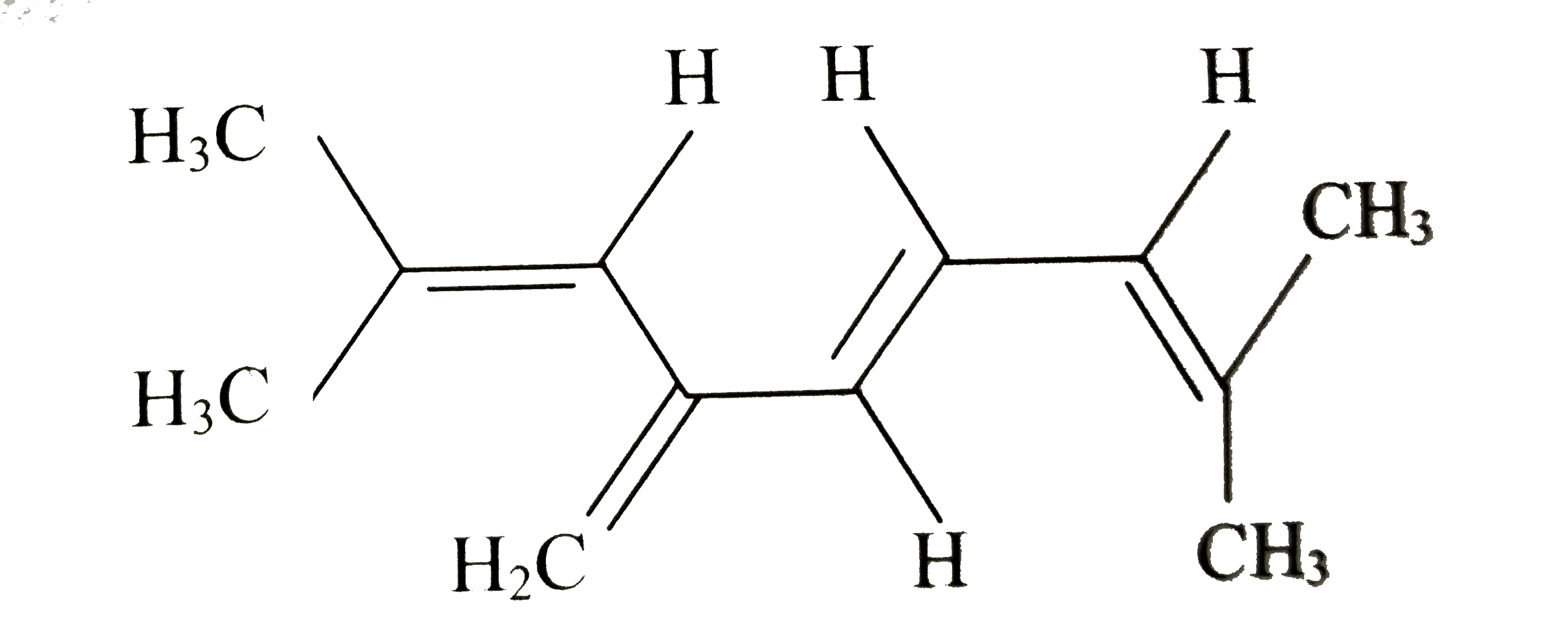
View SolutionNumber of π bonds and σ bonds in the following structure is
View SolutionThe total number of π-bond electrons in the following structure is
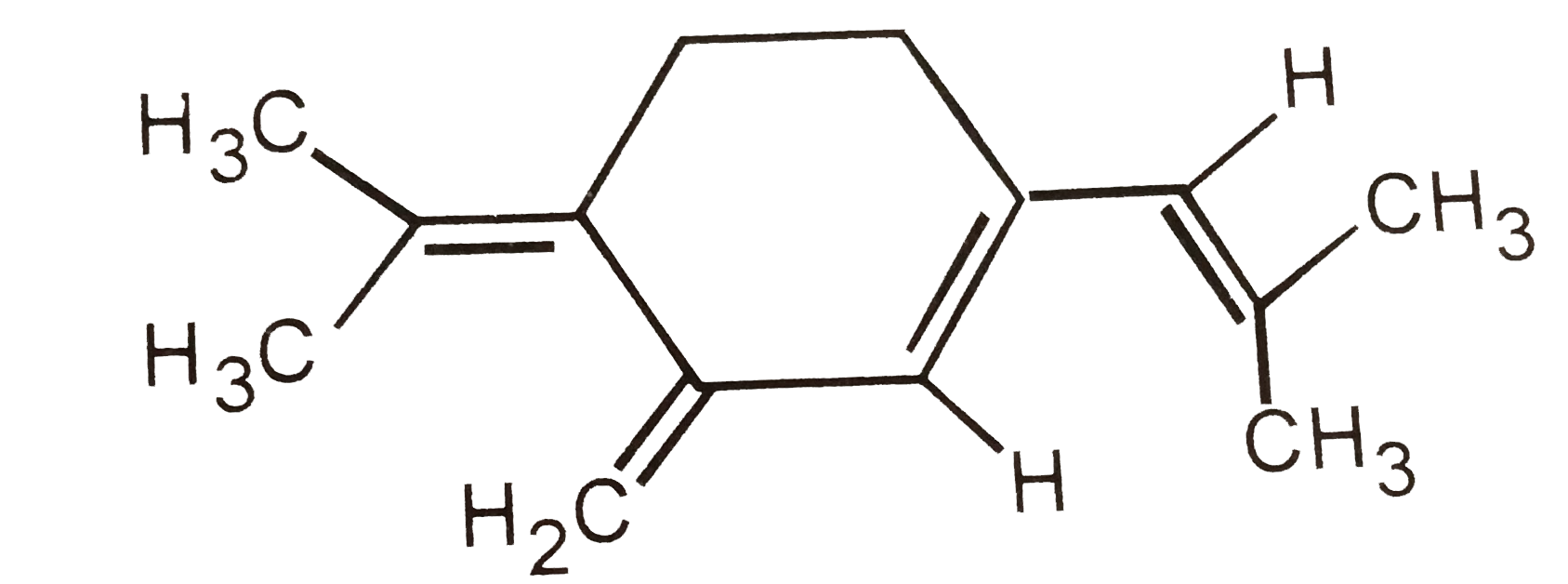
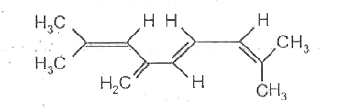
View SolutionThe total number of pi - bond electrons in the following structure is
View SolutionNumber of pi bonds and sigma bonds in the following structure is
View SolutionNumber of pi bonds and sigma bonds in the following structure is
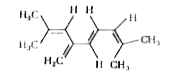
View SolutionThe total number of π-bond electrons in the following structure is
View Solutionनिम्नलिखित संरचना में कुल π -आबन्ध इलेक्ट्रॉनों की संख्या है
View SolutionNumber of π bonds and σ bonds in the following structure is :
View Solution

