Which of the following alkane has lowest boiling point and highest melting point ?
A
n-Pentane
B
Isopentane
C
Neopentane
D
n-Hexane
Video Solution
Text Solution
The correct Answer is:C
Due to least surface area, neopentane has the lowest b.p. but due to high symmetry it has the highest m.p.
|
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:TEST YOUR GRIP (FILL IN THE BLANKS )
Explore 20 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:CONCEPTUAL Questions
Explore 28 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:PROBLEMS FOR PRACTICE
Explore 3 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Knowledge Check
Which of the following alkanes has the lowest boiling ?
An-PentaneBIsopentaneCNeopentanoDn-Hexane.Which of the following alkanes will have the lowest boiling point?
An-PentaneBIsopentaneCNeopentaneDn-Hexane.Which of the following isomer has lowest boiling point ?
A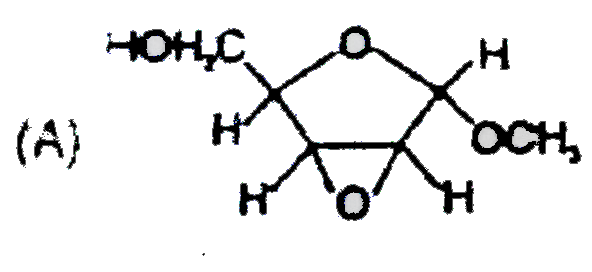 B
B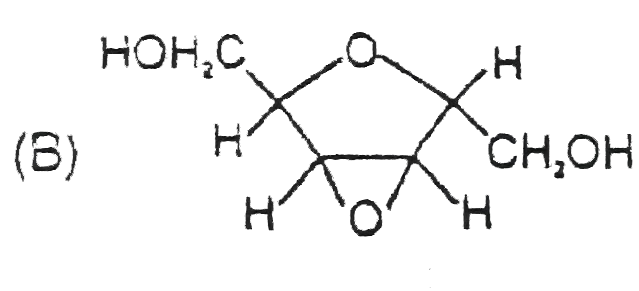 C
C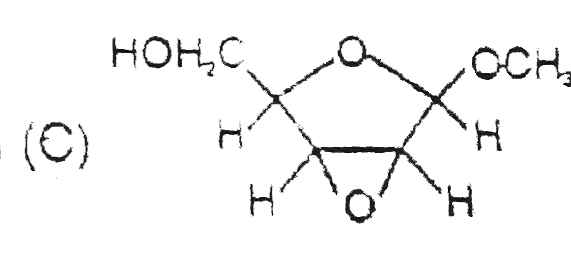 D
D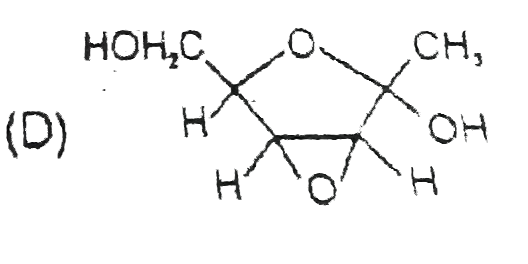
Which of the following has the lowest boiling points?
View SolutionWhich of the following has lowest boiling point ?
View SolutionWhich of the following has lowest boiling point ?
View SolutionWhich of the following compound has lowest boiling point?
View SolutionWhich of the follwing alkanes has the lowest melting point?
View Solution
