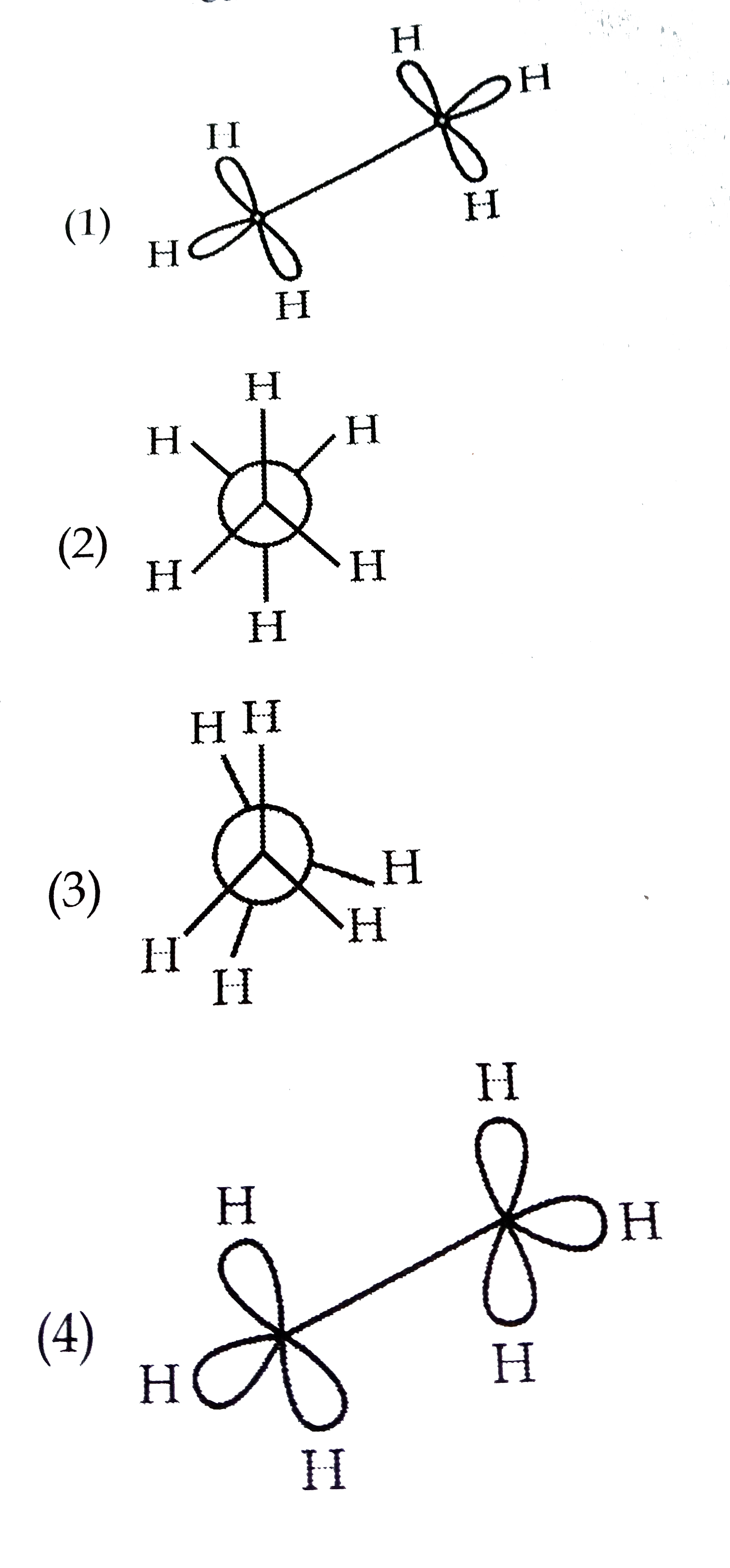
The dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is
Video Solution
Text Solution
The correct Answer is:60∘
|
Answer
Step by step video & text solution for The dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:CONCEPTUAL Questions
Explore 28 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT QUESTION AND EXERCISES WITH ANSWERS
Explore 13 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:TEST YOUR GRIP (MULTIPLE CHOICE )
Explore 26 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Knowledge Check
The dihedral angle between the hydrogen atoms of two methyl groups is staggered conformation of ethane is
A120∘B180∘C90∘D60∘The dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is
A0∘B60∘C120∘D240∘The dihedral angle between the hydrogen atoms of two methyl groups in staggered conformation of ethane is
A0∘B60∘C120∘D240∘
The eclipsed and staggered conformation of ethane is due to -
View SolutionThe eclipsed and staggered conformation of ethane is due to -
View SolutionDihedral angle in staggerred form of ethane is
View SolutionThe dihedral angle between two methyl group in partially eclipsed conformation of n-butane is
View SolutionThe dihedral angle between two C-H bonds the staggered conformation of ehtane is
View Solution