Which of the following has the highest boiling point ?
(i) 2-Methylpentane (ii) 2, 3-Dimethylbutane (iii) 2, 2-Dimethylbutane
Video Solution
Text Solution
As the branching increases , surface area decreases. As a result, magnitude of van der Waals forces of attraction decreases and hence the boiling point decreases. Now 2-methylpentane has the largest surface area and hence has the highest boiling point. Further because of the presence of two branches on the same carbon, 2,2-dimethylbutane has lower surface area and hence has lower boiling point than that of 2,3-dimethylbutane. Thus, the overall order of decreasing boiling points is : 2-methylpentane (333 K) gt 2,3- dimethylbutane (331 K) gt 2,2-dimethylbutane (323 K)
|
Topper's Solved these Questions
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - NCERT QUESTION AND EXERCISES WITH ANSWERS
13 Videos
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - NCERT QUESTION AND EXERCISES WITH ANSWERS (NCERT EXCERCISES)
24 Videos
- HYDROCARBONSBOOK - PRADEEPCHAPTER - HYDROCARBONSEXERCISE - TEST YOUR GRIP (FILL IN THE BLANKS )
20 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBOOK - PRADEEPCHAPTER - EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESEXERCISE - Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
10 Videos
- HYDROGEN BOOK - PRADEEPCHAPTER - HYDROGEN EXERCISE - COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
15 Videos
Similar Questions
Knowledge Check
Which among the following has the highest boiling point?
ACH3CH2CH2NH2B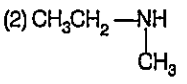 C
C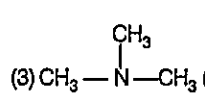 DCH3NH2
DCH3NH2Which of the the following has highest boiling point ?
ACH3NH2BCH3OHCCH3CH3DCH3OCH3Which one of the following has the highest boiling point ?
AH2OBH2SCH2SeDH2Te
Which of the following has highest boiling point?
View SolutionWhich of the following has highest boiling point ?
View SolutionWhich of the following has highest boiling point ?
View Solutionwhich of the following has highest boiling point ?
View SolutionWhich of the following has highest boiling point ?
View Solution

