Which of the following are correct ?
CH3−O−CH⊕2 is more stable than CH3−CH⊕2
(CH3)2CH⊕ is less stable than CH3−CH2−CH⊕2
CH2=CH−CH⊕2 is more stable than CH3−CH2−CH⊕2
CH2=CH⊕ is more stable than CH3−CH⊕2
The correct Answer is:A, C
To determine which statements regarding carbocation stability are correct, we can analyze the concepts of stability in carbocations, including the effects of mesomeric, hyperconjugation, inductive, and resonance effects.
1. Understanding Carbocation Stability:
- Carbocations are positively charged carbon species. Their stability is influenced by the surrounding groups and the type of bonds they form. The more stable a carbocation is, the more readily it can exist.
2. Evaluating Aromaticity:
- The first point mentions aromaticity. Aromatic compounds are cyclic, planar, and follow Huckel's rule (4n + 2 π electrons). Since carbocations are not inherently aromatic, this point is false.
3. Mesomeric Effect:
- The second point discusses the mesomeric effect, which involves the delocalization of electrons. Carbocations can be stabilized by adjacent double bonds or lone pairs that can donate electron density. Thus, this point is true.
4. Inductive and Hyperconjugation Effects:
- The next points involve inductive effects (electron-withdrawing or donating effects through sigma bonds) and hyperconjugation (stabilization due to the overlap of σ-bonds with an empty p-orbital).
- Comparing two carbocations, one with more alkyl groups (which can donate electron density through hyperconjugation) will be more stable than one with fewer alkyl groups. Therefore, if we have CH3^+ vs. CH2^+CH3, the CH3^+ is less stable due to less hyperconjugation. This point is false.
5. Comparing SP2 and SP3 Hybridization:
- The stability of carbocations also depends on hybridization. SP2 hybridized carbocations (like those with double bonds) are generally more stable than SP3 hybridized ones. If the statement indicates that SP2 carbocations are less stable than SP3, it is false.
6. Conclusion:
- Based on the analysis, the correct statements are those related to mesomeric effects and the general understanding of carbocation stability. The statements regarding aromaticity, hyperconjugation, and hybridization comparisons are incorrect.
Final Answer:
- The correct statements are related to the mesomeric effect and the general stability of carbocations. Therefore, options regarding mesomeric effects are true, while those regarding aromaticity and hybridization comparisons are false.
- Carbocations are positively charged carbon species. Their stability is influenced by the surrounding groups and the type of bonds they form. The more stable a carbocation is, the more readily it can exist.
- The first point mentions aromaticity. Aromatic compounds are cyclic, planar, and follow Huckel's rule (4n + 2 π electrons). Since carbocations are not inherently aromatic, this point is false.
- The second point discusses the mesomeric effect, which involves the delocalization of electrons. Carbocations can be stabilized by adjacent double bonds or lone pairs that can donate electron density. Thus, this point is true.
- The next points involve inductive effects (electron-withdrawing or donating effects through sigma bonds) and hyperconjugation (stabilization due to the overlap of σ-bonds with an empty p-orbital).
- Comparing two carbocations, one with more alkyl groups (which can donate electron density through hyperconjugation) will be more stable than one with fewer alkyl groups. Therefore, if we have CH3^+ vs. CH2^+CH3, the CH3^+ is less stable due to less hyperconjugation. This point is false.
- The stability of carbocations also depends on hybridization. SP2 hybridized carbocations (like those with double bonds) are generally more stable than SP3 hybridized ones. If the statement indicates that SP2 carbocations are less stable than SP3, it is false.
- Based on the analysis, the correct statements are those related to mesomeric effects and the general understanding of carbocation stability. The statements regarding aromaticity, hyperconjugation, and hybridization comparisons are incorrect.
- The correct statements are related to the mesomeric effect and the general stability of carbocations. Therefore, options regarding mesomeric effects are true, while those regarding aromaticity and hybridization comparisons are false.
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (SHORT ANSWER QUESTIONS)
Explore 20 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MATCHING TYPE QUESTIONS)
Explore 4 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE -I)
Explore 9 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Why do alkenes prefer to undergo electrophilec addition reaction while arenes prefer electrophilic substitution reactions ? Explain.
View SolutionMatch the reagent from Column I which on reaction with CH3−CH=CH2 gives some product given in Column II as per the codes given below
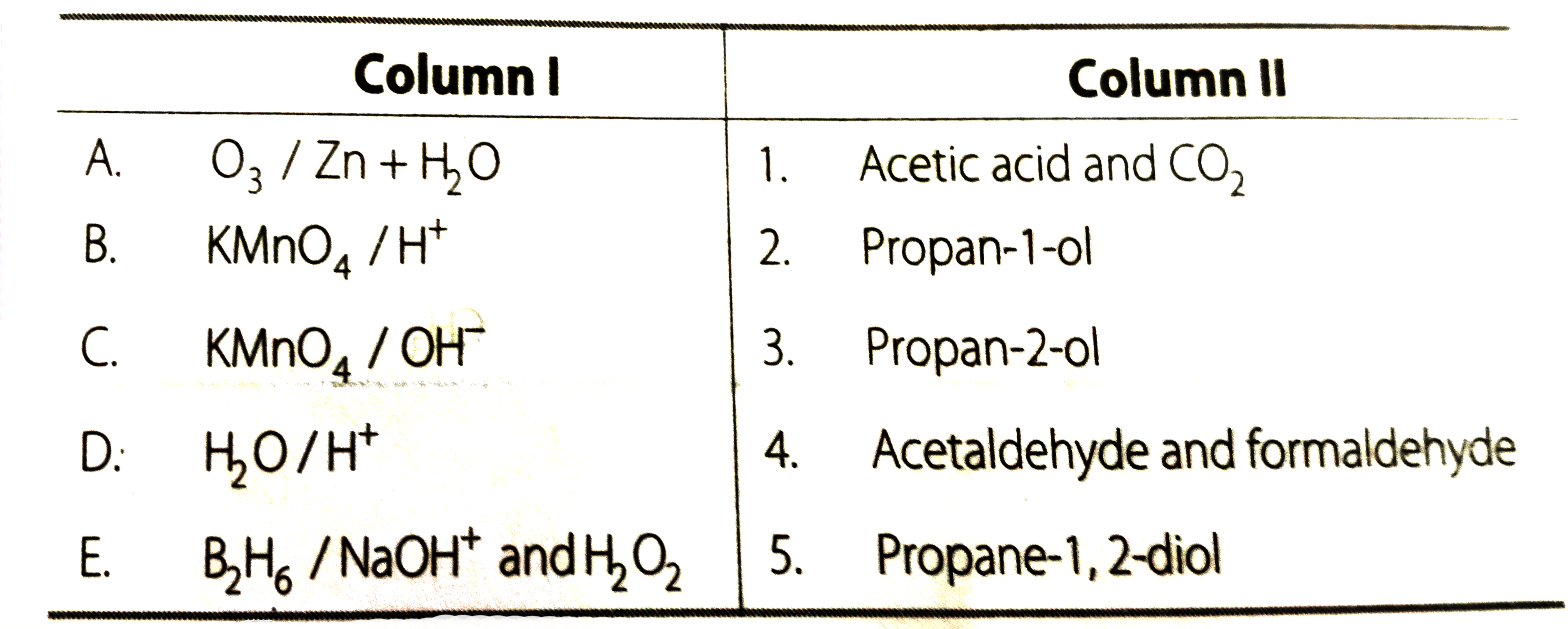
View SolutionArrange the following in decreasing order of their boiling points.
(A). N-butane
(B). 2-methylbutane
(C). N-pentane
(D). 2,2-dimethylpropane
View SolutionIn each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer as
Assertion . The vapour pressure of a pure liquid has a fixed value at a particular temperature .
Reason . When equilibrium is reached , no more vapour are formed .
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View SolutionWhich of the following statements is/are correct about the following is/are correct about the following reactions ?
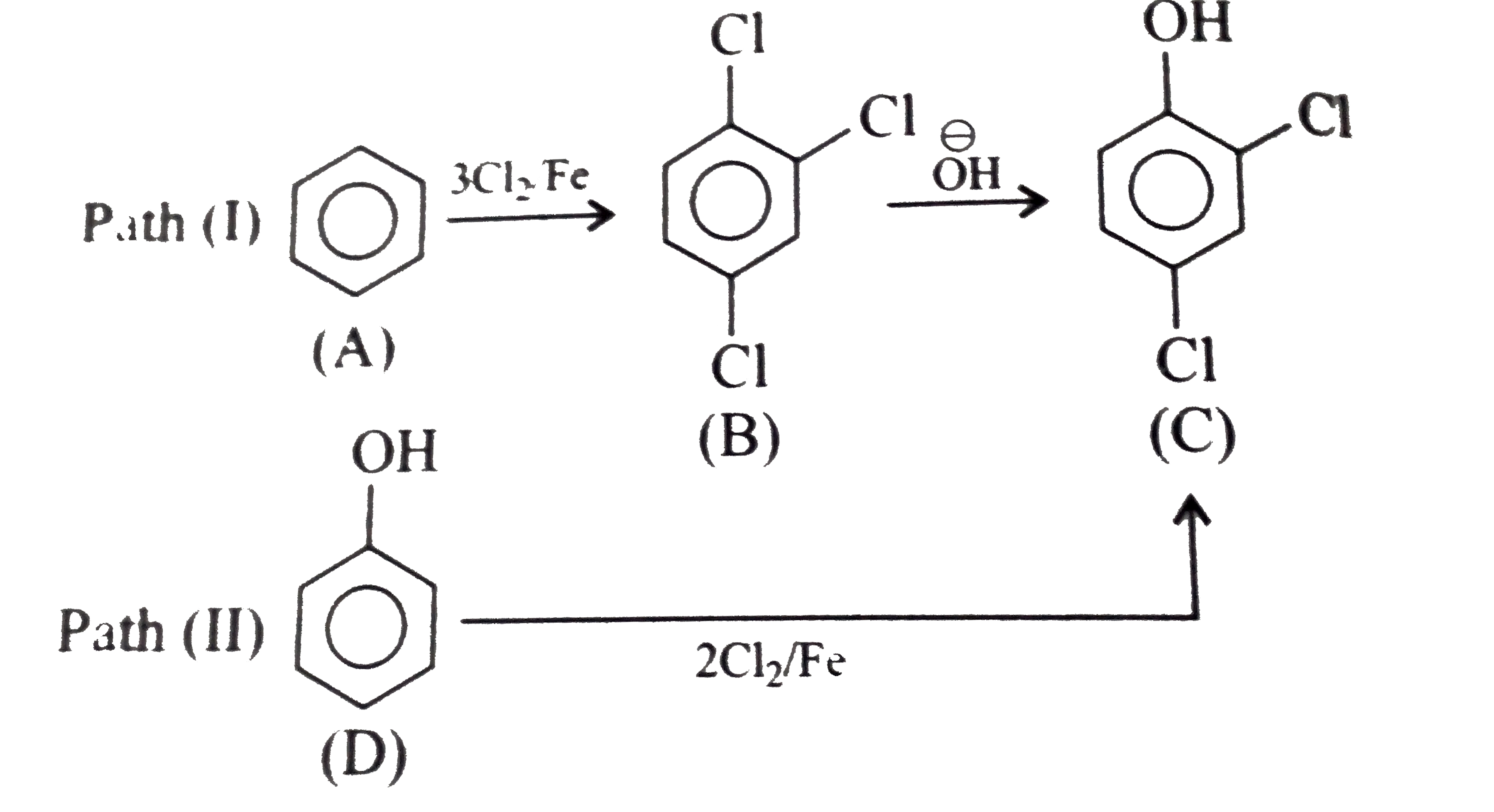
View SolutionWhich of the following are correct reactions ?
View SolutionWhich of the following is not correct ?
View SolutionWhich of the following statement is not correct ?
View SolutionWhich of the following statement is/are correct ?
View SolutionWhich of the following statement is correct ?
View Solutionगेटो के निम्न संयुग्मन के लिए कौन-सी सत्यता सारणी सही है -
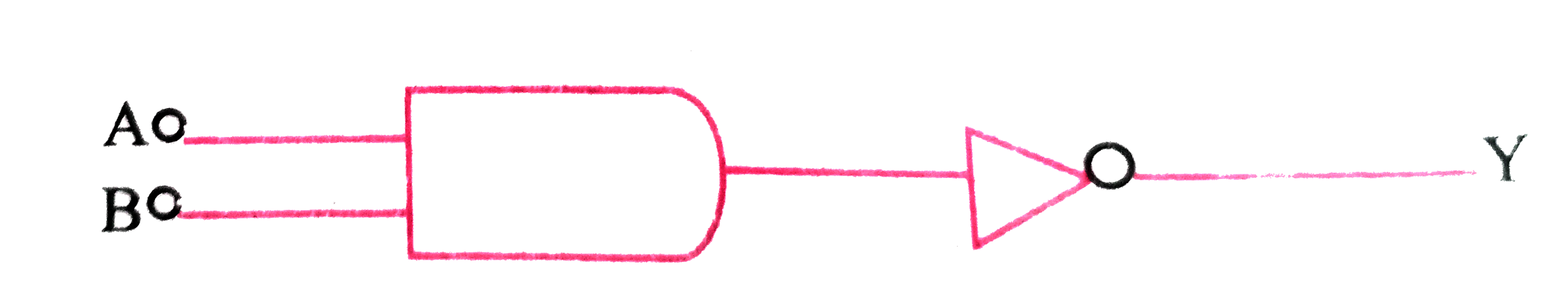
View SolutionWhich of the following is not correct?
View SolutionWhich of the following statements are correct ?
View SolutionMultiple correct postmodern Which of the following statements is correct?
View SolutionChoose the correct option.
Which is following relations is correct?
View Solution
