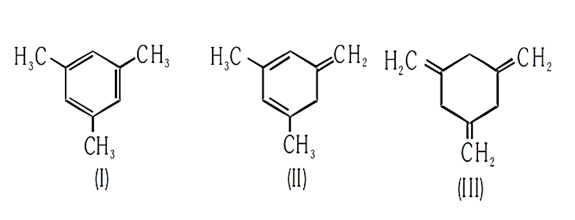
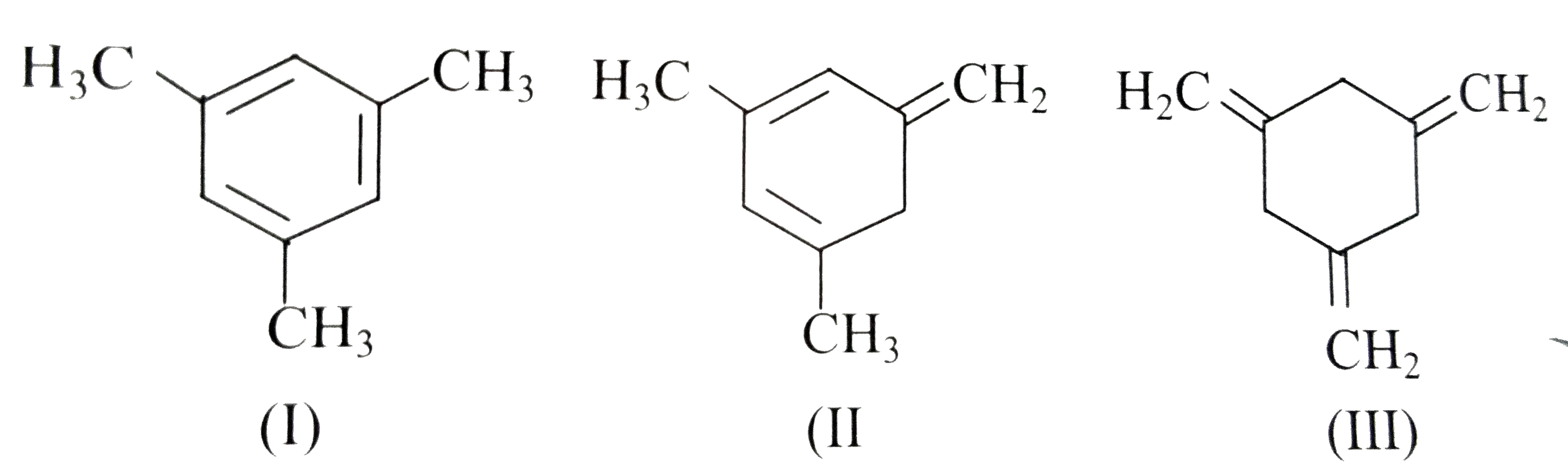
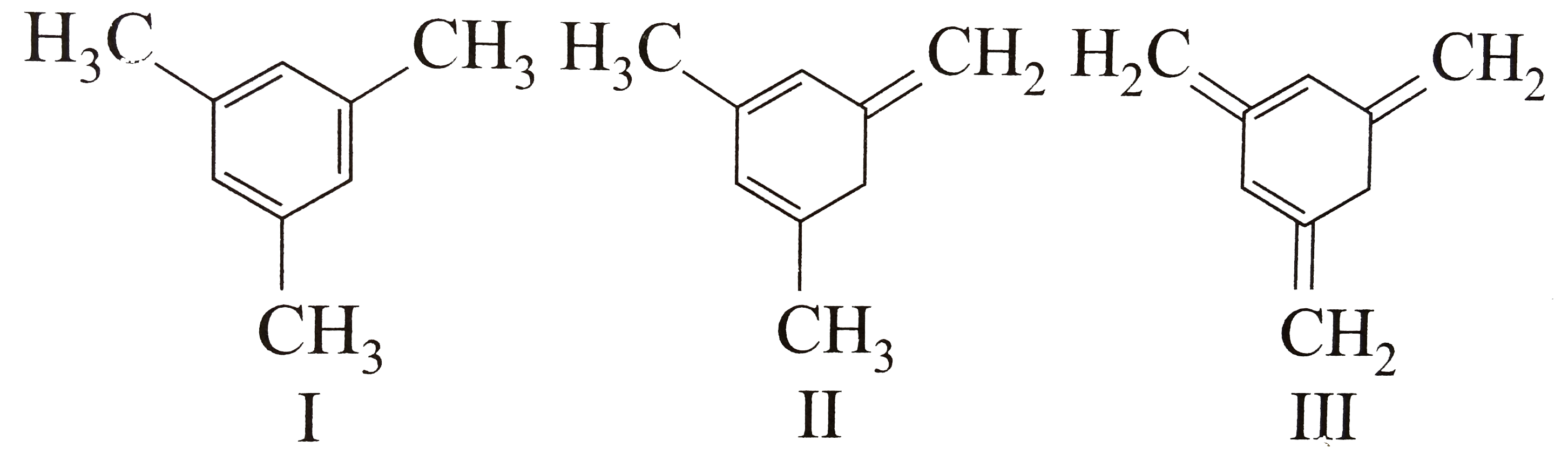
Given :
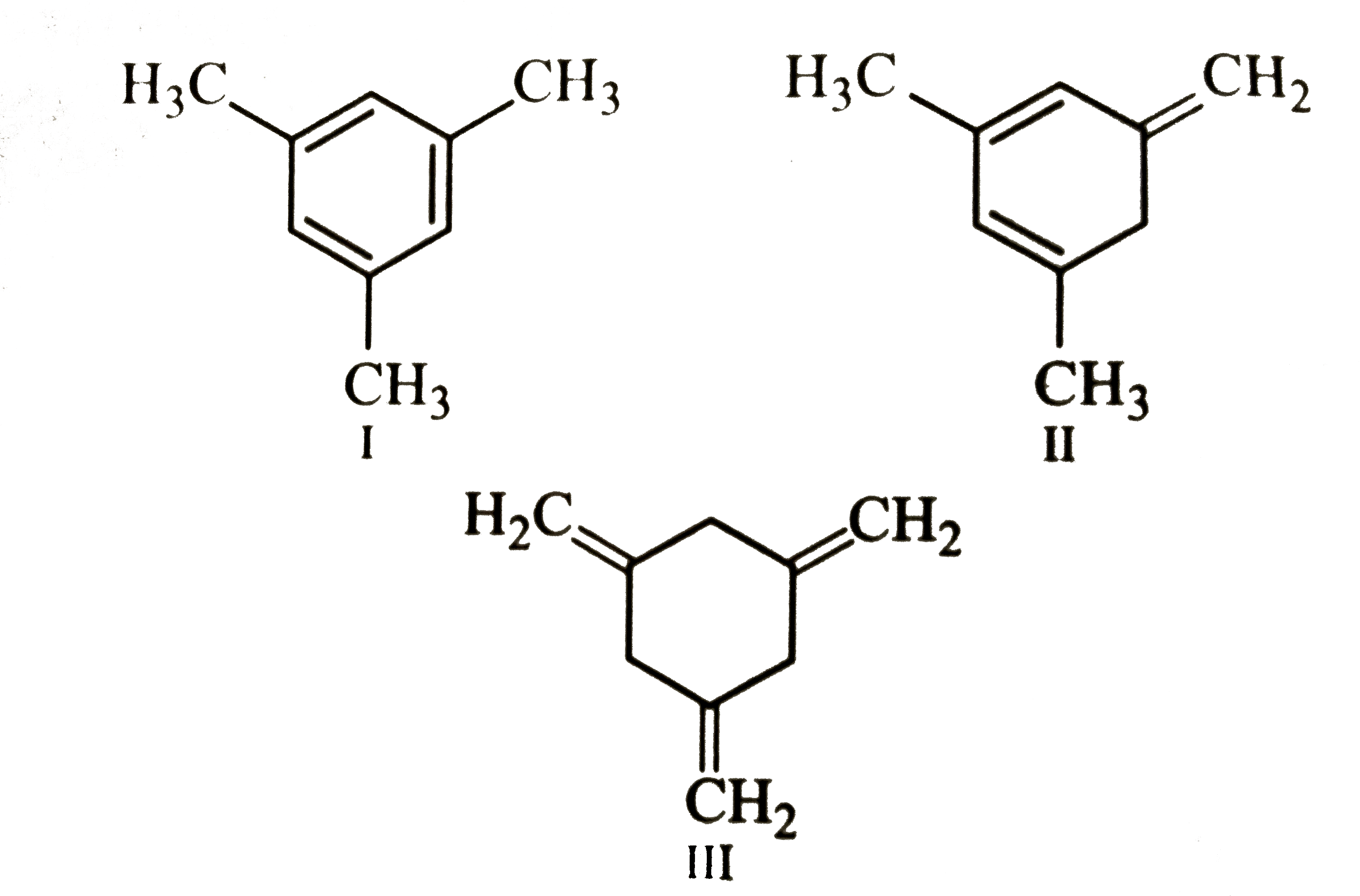
The enthalpy of hydrogenation of these compounds will be in the order is
A
II gt III gt I
B
II gt I gt III
C
I gt II gt III
D
III gt II gt I
Video Solution
Text Solution
The correct Answer is:D
Out of the three compounds (I,II,III), compound (I) is aromatic and hence is most stable. Compound (II) is less stable than compound (I) because it is only a cyclic conjugated diene while compound (III) is least stable since it is neither an aromatic compound nor a cyclic conjugated diene. Thus, the stability of alkenes decreases in the order : I gt II gt III.
Since enthalpy of hydrogenation is inversely proportional to its stability, therefore, enthalpy of hydrogenation decreases in the order : III gt II gt I , i.e., option (d) is correct.
|
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) II. MULTIPLE CHOICE
Explore 13 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE
Explore 14 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 2 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Knowledge Check
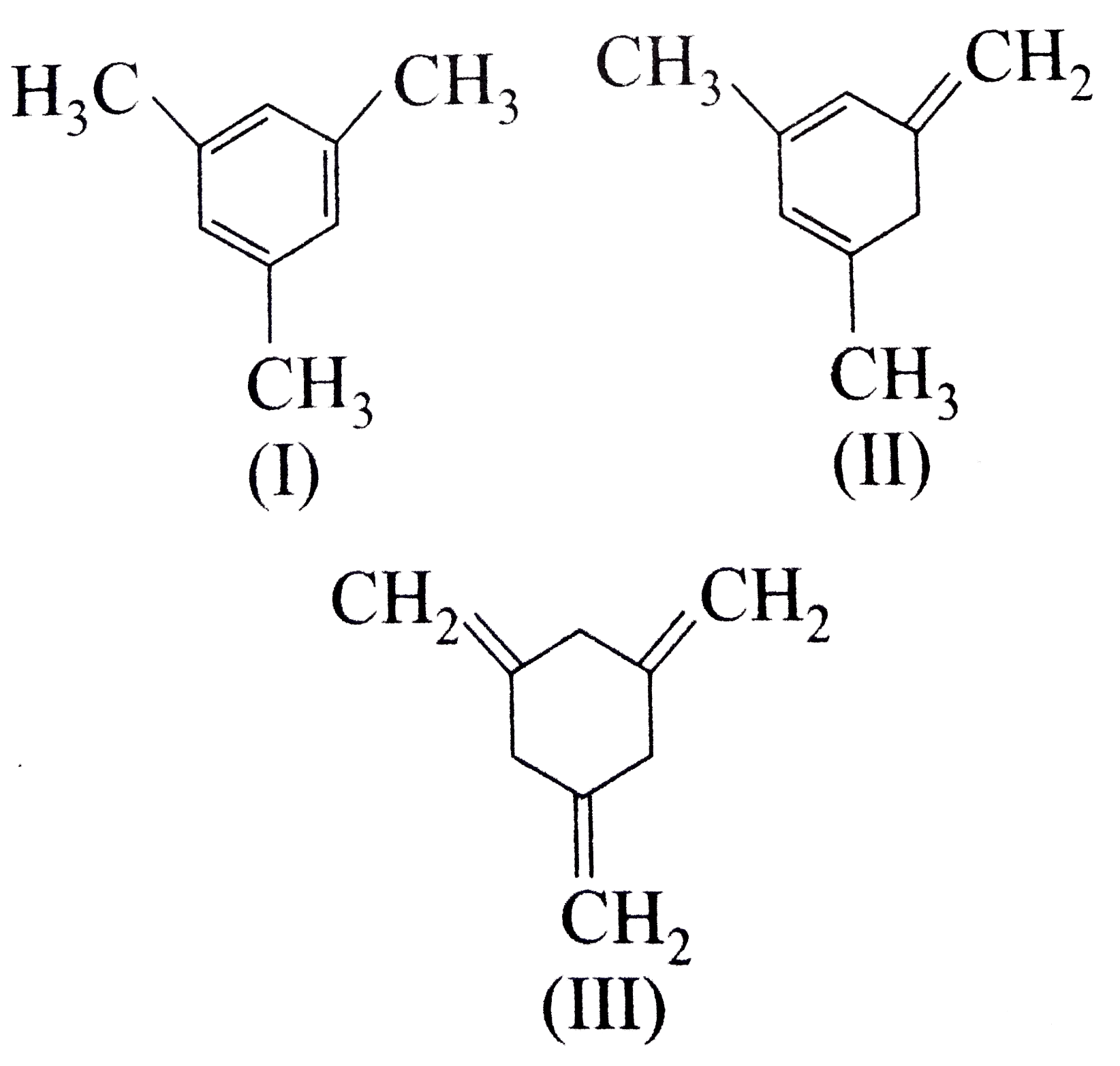
Given :
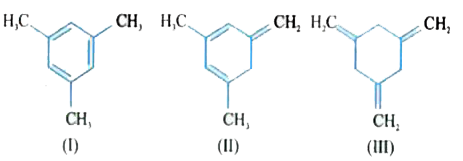
The enthalpy of hydrogenation of these compounds will be in the order as :AII gt III gt IBII gt I gt IIICI gt II gt IIIDIII gt II gt IGiven :
The enthalpy of hydrogenation of these compounds will be in the order asAII>III>IBII>I>IIICI>II>IIIDIII>II>IGiven :
The enthalpy of the hydrogenation of these compounds will be in the order as :AIII>II>IBII>III>ICII>I>IIIDI>II>III
The basic nature of given compounds will be in order:
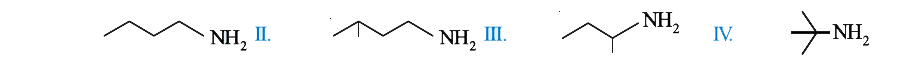
View SolutionEnthalpy of formation of a compound is equal in magnitude to
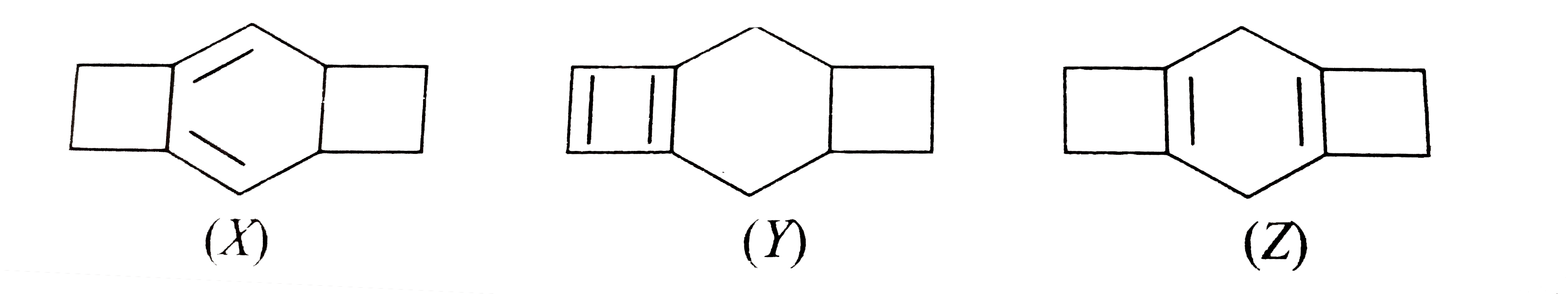
View SolutionFind out correct order for the heat of hydrogenation of these compounds :
View SolutionEnthalpy of formation of compound is
View SolutionStandard enthalpy of formation of compound is defined as
View Solution