Which of the following compounds on decarboxylation with soda-lime will give toluene ?
Phenylacetic acid
o-Toluic acid
m-Toluic acid
p-Toluic acid
The correct Answer is:A, B, C, D
To determine which compound, when subjected to decarboxylation with soda-lime, will yield toluene, we need to analyze the given compounds. The decarboxylation reaction involves the removal of a carboxyl group (-COOH) from a carboxylic acid, resulting in the formation of a hydrocarbon.
1. Identify the Compounds: The compounds under consideration are phenylacetic acid, ortho-toluic acid, meta-toluic acid, and para-toluic acid.
2. Understand Decarboxylation: Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). When a carboxylic acid undergoes decarboxylation with soda-lime (a mixture of sodium hydroxide and calcium oxide), it typically yields an alkane.
3. Analyze Each Compound:
- Phenylacetic Acid (C6H5-CH2-COOH): Upon decarboxylation, the carboxyl group is removed, resulting in toluene (C6H5-CH3).
- Ortho-Toluic Acid (C6H4(CH3)(COOH)): Decarboxylation will remove the -COOH group, leaving behind ortho-xylene, which is not toluene.
- Meta-Toluic Acid (C6H4(CH3)(COOH)): Similar to ortho-toluic acid, decarboxylation will yield meta-xylene, not toluene.
- Para-Toluic Acid (C6H4(CH3)(COOH)): Decarboxylation will lead to para-xylene, which is also not toluene.
4. Conclusion: The only compound that, upon decarboxylation with soda-lime, yields toluene is Phenylacetic Acid.
Final Answer:
The compound that will give toluene upon decarboxylation with soda-lime is Phenylacetic Acid.
---
- Phenylacetic Acid (C6H5-CH2-COOH): Upon decarboxylation, the carboxyl group is removed, resulting in toluene (C6H5-CH3).
- Ortho-Toluic Acid (C6H4(CH3)(COOH)): Decarboxylation will remove the -COOH group, leaving behind ortho-xylene, which is not toluene.
- Meta-Toluic Acid (C6H4(CH3)(COOH)): Similar to ortho-toluic acid, decarboxylation will yield meta-xylene, not toluene.
- Para-Toluic Acid (C6H4(CH3)(COOH)): Decarboxylation will lead to para-xylene, which is also not toluene.
The compound that will give toluene upon decarboxylation with soda-lime is Phenylacetic Acid.
Topper's Solved these Questions
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) III. MULTIPLE CHOICE
Explore 14 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) IV. MATCHING TYPE QUESTIONS
Explore 3 Videos
- HYDROCARBONSBook:PRADEEPChapter:HYDROCARBONSExercise:Competition Focus (JEE(main and advanced)/Medical Entrance) I. MULTIPLE CHOICE QUESTIONS
Explore 179 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
- HYDROGEN Book:PRADEEPChapter:HYDROGEN Exercise:COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
Explore 15 Videos
Similar Questions
Catalytic hydrogenation involves addition of hydrogen to a gt C = Clt or -C ≡ C- bond in presence of a metal catalyst such as Ni, Pt, Pd, etc. These are called heterogeneous catalysts. Both H2 and alkene/alkyne get adsorbed on the surface of the metal , and syn-addition of hydrogen atoms takes place. The relative rates of hydrogenation follows the order:
−C≡C−>>C=C<>C=o<C6H6
If sodium or lithium in liquid ammonia is used, anti-addition of H-atoms occurs across −C≡C−bond. These reducing agents can also be used to reduce double bonds of benzene rings and conjugated dienes but not of isolated dienes.
Which of the following alkenes will react most readily with H2 under catalytic hydrogeneration conditions ?
View SolutionMatch the following columns
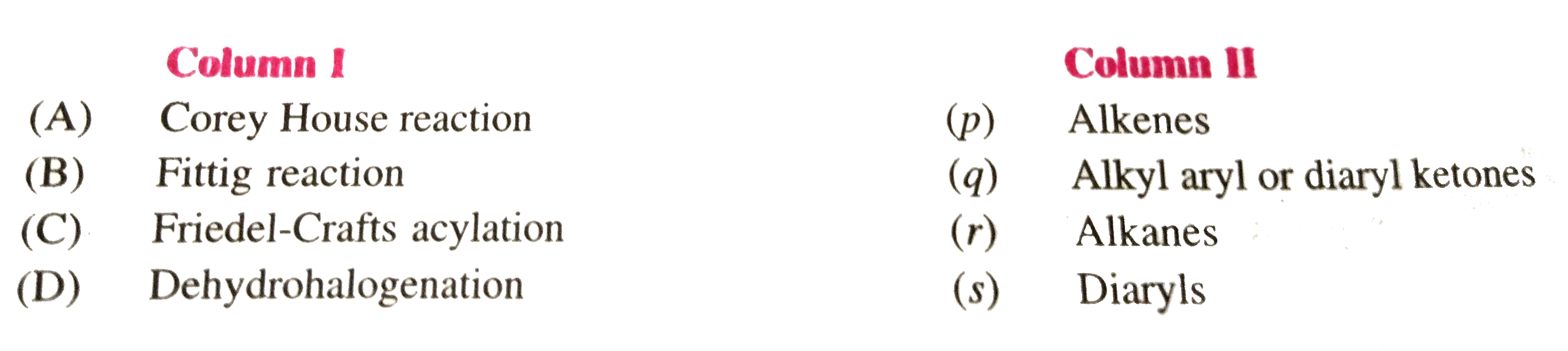
View SolutionPick out the alkane which differs from the other members of the group.
View SolutionIn each of the following questions, a statement of Assertion is given followed by a corresponding statement of Reason just below it. Of the statements, mark the correct answer as
Assertion . The vapour pressure of a pure liquid has a fixed value at a particular temperature .
Reason . When equilibrium is reached , no more vapour are formed .
View SolutionAssertion : The water gas shift reaction can be used to increase the amount of H2 in the shift ' syn gas ' mixture.
Reason : In this reaction, water is reduced to H2 by CO.
View SolutionIn order to get propane gas which of the following should be subjected to soda lime decarboxylation?
View SolutionWhich of the following acid will give isopentane on decarboxylation with soda lime?
View SolutionDecarboxylation of sodium salicylate with soda lime form :
View SolutionWhich of the following is the correct order of decarboxylation of following carboxylic acids using soda lime?
View SolutionDecarboxylation of sodium salicylate with soda lime forms
View SolutionWhich acid will not form hydrocarbon on soda lime decarboxylation ?
View SolutionWhich of following shows highest rate of decarboxylation in presence of soda lime :
View SolutionSoda lime is used extensively in decarboxylation reaction to obtain alkanes. Soda lime is
View SolutionDecarboxylation of sodium benzoate on heating with soda lime gives
View SolutionSoda lime is used extensively in decarboxylation reaction to obtain alkanes. Soda lime is:
View Solution
