The amount of oxygen consumed by pollutants in water found by titration with acidified K2Cr2O7 is called _____ of water.
The correct Answer is:Chemical Oxygen Demand (COD)
To solve the question regarding the amount of oxygen consumed by pollutants in water, we follow these steps:
1. Understanding the Question: The question asks for the term that describes the amount of oxygen consumed by pollutants in water, which is determined through a titration process using acidified potassium dichromate (K2Cr2O7).
2. Identifying the Process: The titration with acidified K2Cr2O7 is a common analytical method used to measure the amount of oxygen that can be consumed by organic and inorganic substances in a sample of water.
3. Defining the Term: The specific term used to describe the amount of oxygen consumed by pollutants in water is known as Chemical Oxygen Demand (COD).
4. Conclusion: Therefore, the answer to the question is that the amount of oxygen consumed by pollutants in water found by titration with acidified K2Cr2O7 is called Chemical Oxygen Demand (COD).
Final Answer:
The amount of oxygen consumed by pollutants in water found by titration with acidified K2Cr2O7 is called Chemical Oxygen Demand (COD).
---
The amount of oxygen consumed by pollutants in water found by titration with acidified K2Cr2O7 is called Chemical Oxygen Demand (COD).
টপার এই প্রশ্নগুলো সমাধান করেছে
- ENVIRONMENTAL CHEMISTRYBOOK - PRADEEPCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - CONCEPTUAL QUESTIONS
8 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - PRADEEPCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - NCERT Questions And Exercises With Answers
20 Videos
- ENVIRONMENTAL CHEMISTRYBOOK - PRADEEPCHAPTER - ENVIRONMENTAL CHEMISTRYEXERCISE - TEST YOUR GRIP (MULTIPLE CHOICE )
8 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES BOOK - PRADEEPCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES EXERCISE - Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
12 Videos
- EQUILIBRIUMBOOK - PRADEEPCHAPTER - EQUILIBRIUMEXERCISE - Competition Focus (VIII. Assertion-Reason Type Questions)
16 Videos
সম্পর্কিত প্রশ্ন
জ্ঞান পরীক্ষা করুন
Methyl alcohol on oxidation with acidified K2Cr2O7 giyes
ACH3COCH3BCH3CHOCHCOOHDCH3COOHMethyl alcohol on oxidation with acidified K2Cr2O7 gives
ACH3OHBHCOOHCCH3COCH3DCH3COOHMethanol on oxidation with acidified K2Cr2O7 gives the product ________
AacetaldehydeBformic acidCacetic acidDacetone
Ethyl alcohol on oxidation by acidified K2Cr2O7 gives
View SolutionIn the reaction with acidified K2Cr2O7 solution ,SO2 acts as
View Solution1-butanol is oxidised by acidified K2Cr2O7 give,
View SolutionK2Cr2O7is NOT used in ____________.
View SolutionIn the oxidation of
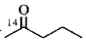 by acidified K2Cr2O7 the product(s) is/are
by acidified K2Cr2O7 the product(s) is/are
View Solution

