The intermediate during the addition of HCl to propene in the presence of peroxide is :
CH3−CH−CH2CI
CH3−+CH−CH3
CH3−CH2−CH2
CH3−CH2−+CH2
The correct Answer is:2
Step by step video, text & image solution for The intermediate during the addition of HCl to propene in the presence of peroxide is : by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- HALOALKANES AND HALOARENES BOOK - AAKASH INSTITUTECHAPTER - HALOALKANES AND HALOARENES EXERCISE - Assignment (Section - B ) ( Objective Type Question(One option is correct ))
37 Videos
- HALOALKANES AND HALOARENES BOOK - AAKASH INSTITUTECHAPTER - HALOALKANES AND HALOARENES EXERCISE - Assignment (Section - C ) ( Objective Type Question(More than one options are correct ))
18 Videos
- HALOALKANES AND HALOARENES BOOK - AAKASH INSTITUTECHAPTER - HALOALKANES AND HALOARENES EXERCISE - Illustration
5 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBOOK - AAKASH INSTITUTECHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSEXERCISE - Try Yourself
33 Videos
- HYDROCARBONSBOOK - AAKASH INSTITUTECHAPTER - HYDROCARBONSEXERCISE - Assignment(Section - D) (Assertion -Reason Type Question)
24 Videos
Similar Questions
The compound C2FCI Brl has
View SolutionWhich of the following cannot be resolved into enantiomers ?
View SolutionDesignate each chirality center as (R ) or (S) in the given compounds
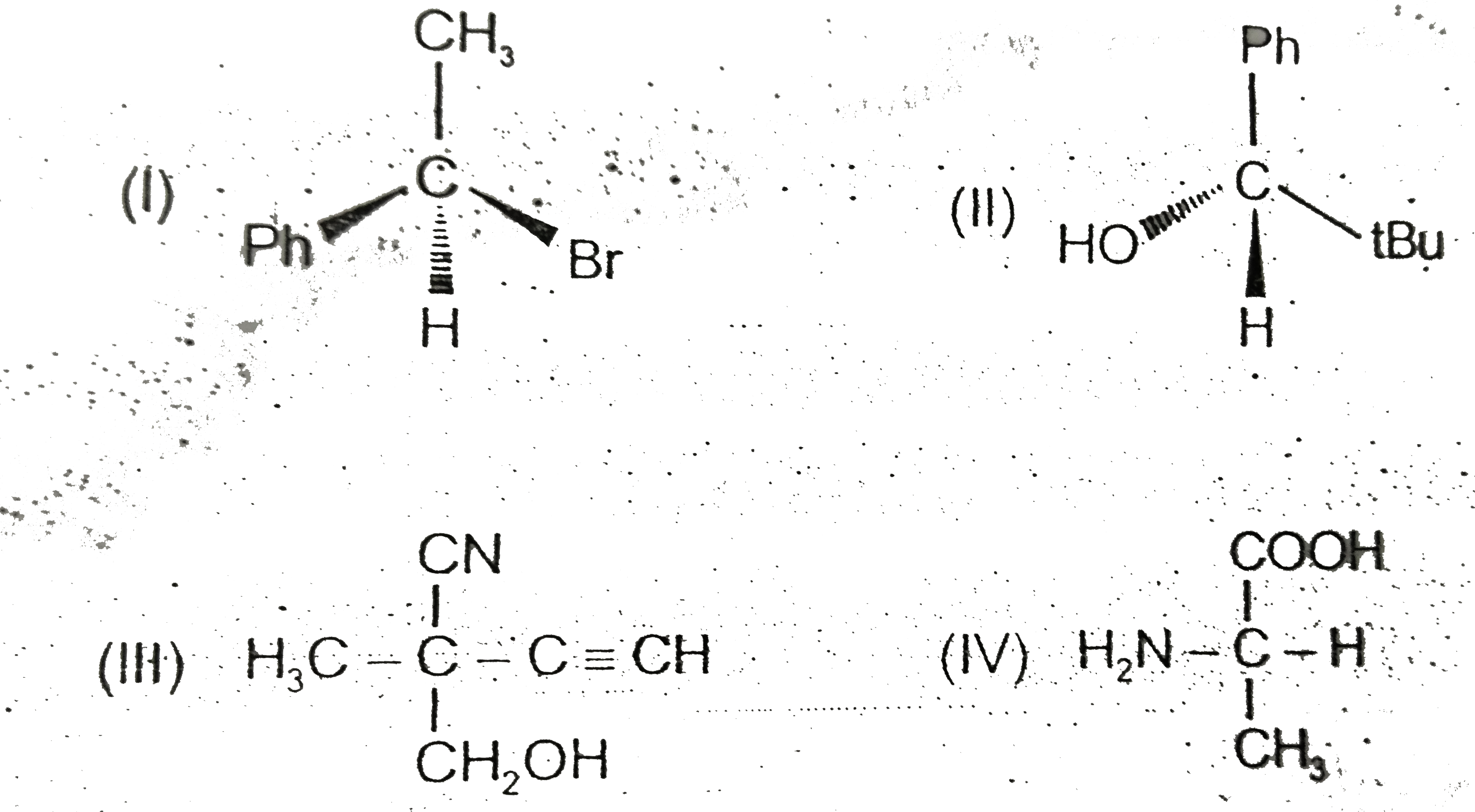
View SolutionMatch the following from column I to column II
Column IColumn IIMalachiteFeSphaleriteAgHorn silverZnChromineCu
View SolutionA : CH≡C−CH2−CH=CH2 adds up HBr to give CH≡C−CH2−C∣BrH−CH3 while CH=C∣Br−CH=CH2 adds up HBr to give CH2=C∣Br−CH=CH2
R : Double bond is always more reactive than triple bond towards electrophillic addition reaction.
View SolutionThe intermediate formed during the addition of HCl to propene in the presence of a peroxide is
View SolutionThe intermediate during the addition of HCl to propene in the presence of peroxide is:
View SolutionThe intermediate during the addition of HCl to propene in the presence of peroxide is :
View SolutionA) Additionof HCl to propene in presence of peroxide gives 1-chloropropane. R) The reaction occurs by carbonium ion intermediate.
View SolutionThe intermediate during the addition of HCl to propene in the presence of peroxide is
View SolutionThe intermediate during the addition of HI to propene in presence of peroxide is _____________.
View SolutionThe intermediate during the addition of HCl to propene in presence of peroxide is
View SolutionThe addition of HBr to propene in the presence of peroxides follows ..........rule.
View SolutionThe intermediate during the addition of HCl to propene is:
View SolutionThe intermediate during the addition of HCl to propene in the presence of peroxide is
View SolutionThe intermediate during the addition of HI to propene in presence of peroxide is _____________.
View Solution

