PhMgBr (Grignard reagent ) cannot be used to prepare the compound ?
The correct Answer is:4
To determine which compound cannot be prepared using phenyl magnesium bromide (Grignard reagent), we will analyze the four options provided.
1. Understanding Grignard Reagents: Grignard reagents, such as phenyl magnesium bromide (PhMgBr), are powerful nucleophiles that can react with electrophiles to form new carbon-carbon bonds. They can react with carbonyl compounds, esters, and other electrophilic species.
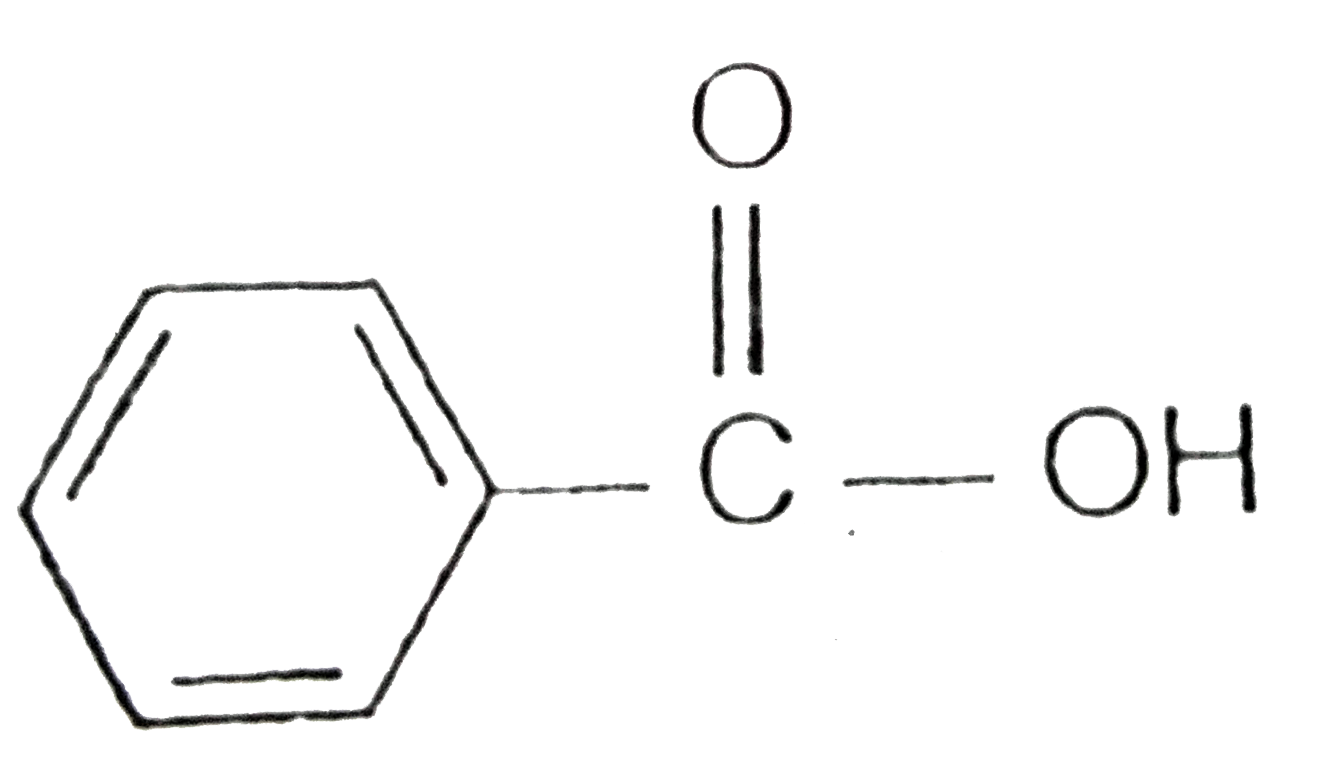
2. Option A: Benzoic Acid
- Reaction: PhMgBr can react with carbon dioxide (CO2) to form benzoic acid. The reaction proceeds as follows:
- PhMgBr + CO2 → PhCOOMgBr (Grignard adduct)
- Hydrolysis of the adduct yields benzoic acid (PhCOOH).
- Conclusion: Benzoic acid can be synthesized using PhMgBr.
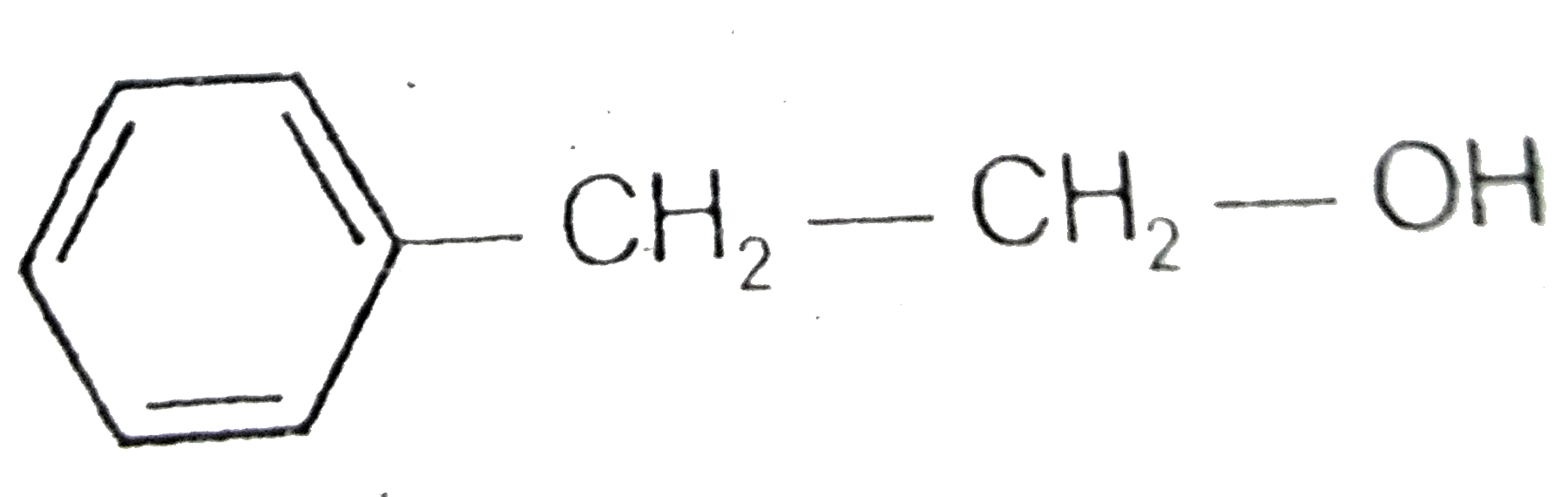
3. Option B: 2-Phenyl Ethanol
- Reaction: PhMgBr can react with an epoxide (oxirane) to open the ring and form 2-phenyl ethanol:
- PhMgBr + Epoxide → Grignard adduct
- Hydrolysis of the adduct yields 2-phenyl ethanol.
- Conclusion: 2-Phenyl ethanol can be synthesized using PhMgBr.
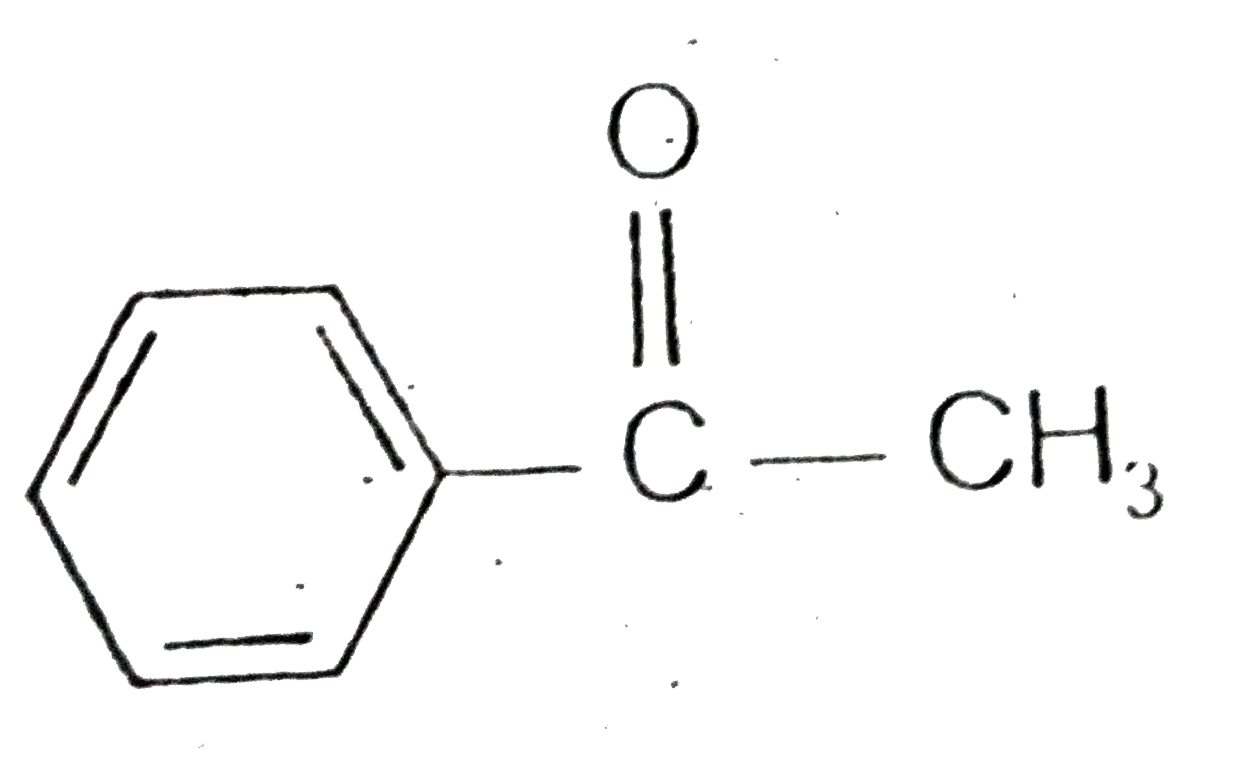
4. Option C: Acetophenone
- Reaction: PhMgBr can react with acetyl chloride (CH3COCl) to form acetophenone:
- PhMgBr + CH3COCl → Grignard adduct
- Hydrolysis of the adduct yields acetophenone (PhCOCH3).
- Conclusion: Acetophenone can be synthesized using PhMgBr.
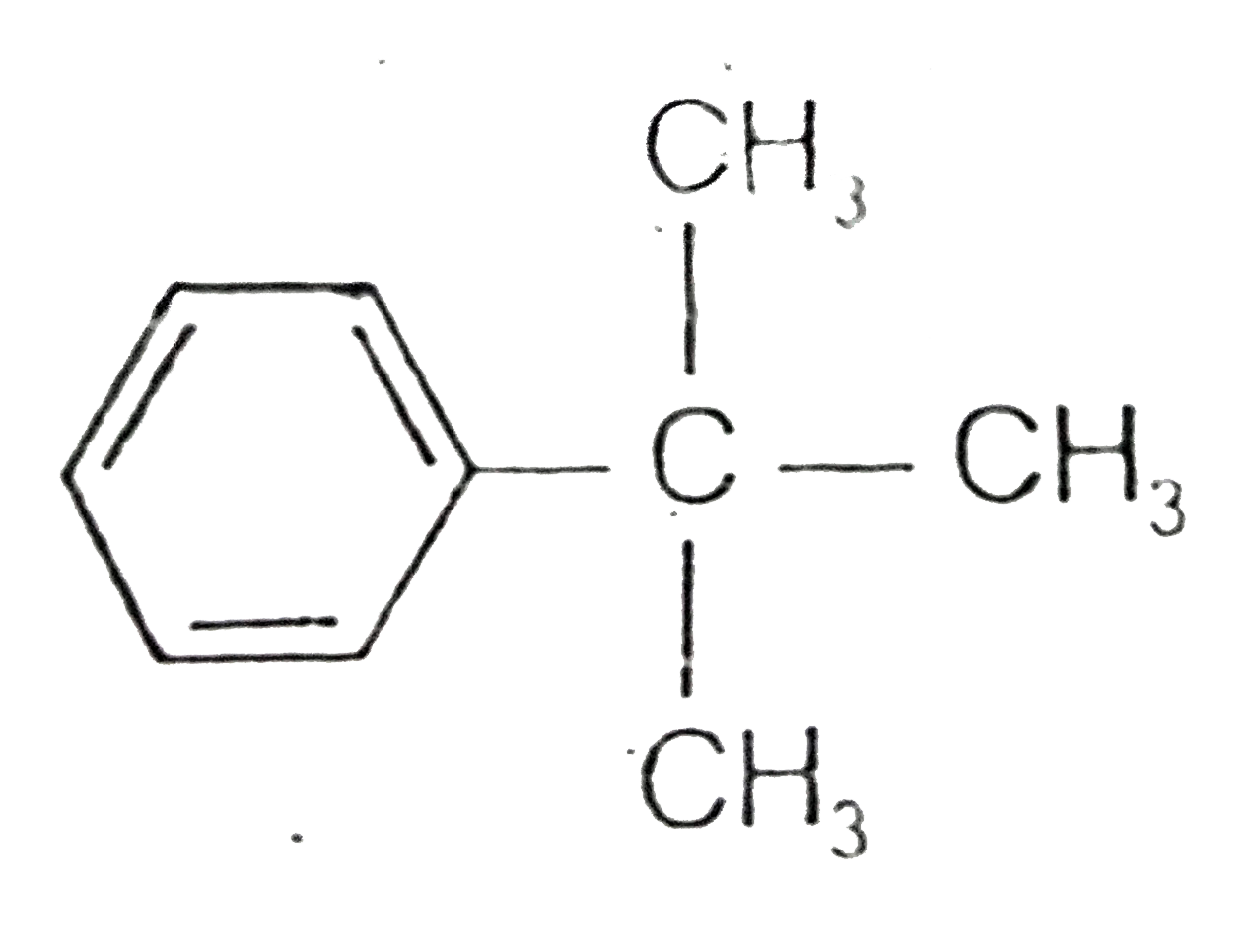
5. Option D: Tertiary Butyl Benzene
- Reasoning: Tertiary butyl benzene (t-BuPh) cannot be synthesized using PhMgBr because:
- Tertiary butyl groups (t-Bu) are not electrophilic enough to react with the nucleophilic Grignard reagent.
- The reaction would require a stable carbocation, which is not feasible in this case.
- Conclusion: Tertiary butyl benzene cannot be synthesized using PhMgBr.
Final Answer:
The compound that cannot be prepared using phenyl magnesium bromide (Grignard reagent) is Tertiary Butyl Benzene (Option D).
---
- Reaction: PhMgBr can react with carbon dioxide (CO2) to form benzoic acid. The reaction proceeds as follows:
- PhMgBr + CO2 → PhCOOMgBr (Grignard adduct)
- Hydrolysis of the adduct yields benzoic acid (PhCOOH).
- Conclusion: Benzoic acid can be synthesized using PhMgBr.
- Reaction: PhMgBr can react with an epoxide (oxirane) to open the ring and form 2-phenyl ethanol:
- PhMgBr + Epoxide → Grignard adduct
- Hydrolysis of the adduct yields 2-phenyl ethanol.
- Conclusion: 2-Phenyl ethanol can be synthesized using PhMgBr.
- Reaction: PhMgBr can react with acetyl chloride (CH3COCl) to form acetophenone:
- PhMgBr + CH3COCl → Grignard adduct
- Hydrolysis of the adduct yields acetophenone (PhCOCH3).
- Conclusion: Acetophenone can be synthesized using PhMgBr.
- Reasoning: Tertiary butyl benzene (t-BuPh) cannot be synthesized using PhMgBr because:
- Tertiary butyl groups (t-Bu) are not electrophilic enough to react with the nucleophilic Grignard reagent.
- The reaction would require a stable carbocation, which is not feasible in this case.
- Conclusion: Tertiary butyl benzene cannot be synthesized using PhMgBr.
The compound that cannot be prepared using phenyl magnesium bromide (Grignard reagent) is Tertiary Butyl Benzene (Option D).
Topper's Solved these Questions
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:Assignment (Section - C ) ( Objective Type Question(More than one options are correct ))
Explore 18 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:Assignment (Section - D) (Linked Comprehension Type Question )
Explore 9 Videos
- HALOALKANES AND HALOARENES Book:AAKASH INSTITUTEChapter:HALOALKANES AND HALOARENES Exercise:Assignment (Section - A ) ( Competition Level Question )
Explore 50 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSBook:AAKASH INSTITUTEChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTSExercise:Try Yourself
Explore 33 Videos
- HYDROCARBONSBook:AAKASH INSTITUTEChapter:HYDROCARBONSExercise:Assignment(Section - D) (Assertion -Reason Type Question)
Explore 24 Videos
Similar Questions
Knowledge Check
Grignard reagent cannot be prepared from
Apropyl bromideBmethyl bromideCpropargyl bromideDAll of theseGrignard reagent can be prepared in :
ADiethyl etherBAlcoholCBenzeneDNone of these.Grignard reagent can be prepared by
ACH3−CH2−CI+Mgdry−−→etherBCH3−CH∣CI−CH2∣OH+Mgdry−−→etherCCH3−CH3∣C∣CH3−OH+Mgdry−−→etherDAll of them
Which reagent cannot be used to prepare an alkyl halide from an alcohol?
View SolutionGrignard reagents are prepared in
View SolutionWhich of the following reagents can be used to prepare an alkyl halide from an alcohol?
View SolutionGrignard reagents are singma- bonded organometallic compound. There exists covalent bond between carbon and magnesium atoms. Grignard reagent finds applications in the sysnthesis of variety of compounds. Gringnard reagent reacts as carbanion and the reaction of carbanion with the proton of an acid is acid- base reaction. Cabonyl compound (including ester) on interation with grignard reagent generates alkoxide ion and thus can be converted into alcohols. Grignard reagent react with almost all functional groups. Notable exceptions are tertiary amines aliphatic and aromatic C=C bonds.
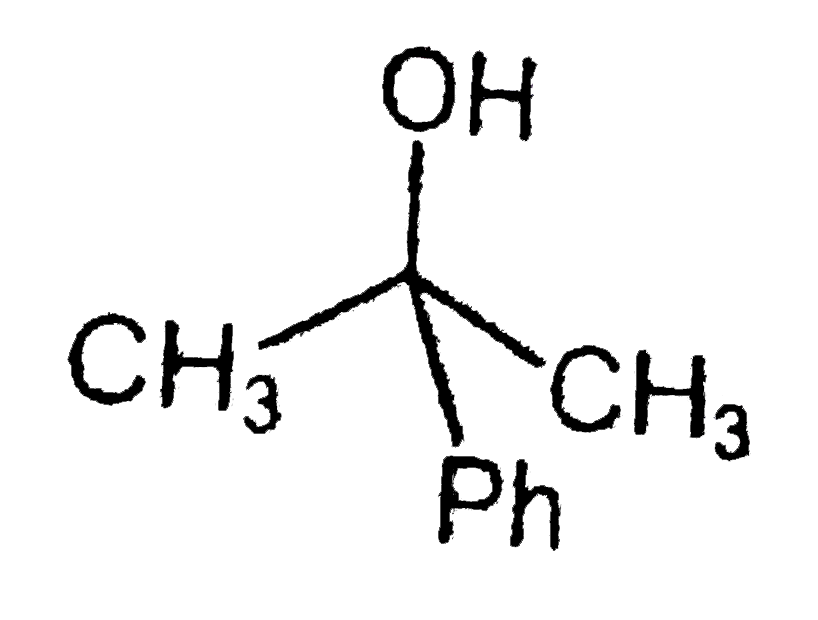
Q. Which of the following combination of reactant can be used to prepare the following given compound?
View SolutionWhich of the following reagents cannot be used for the preparation of hydrogen peroxide ?
View Solution
