−NH2 group is a strong activator towards aromatic electrophilic substiution reaction. Activating capability of −NH2 group can be reduced by treating with
CH3I
CH3O∣∣C−Cl
CH3−NH2
CH3−O∣∣C−O−O∣∣C−CH3
The correct Answer is:B, D
Step by step video & text solution for -NH_(2) group is a strong activator towards aromatic electrophilic substiution reaction. Activating capability of -NH_(2) group can be reduced by treating with by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- AMINES Book:AAKASH INSTITUTEChapter:AMINES Exercise:Assignment Section D Linked Comprehension Type Questions
Explore 14 Videos
- AMINES Book:AAKASH INSTITUTEChapter:AMINES Exercise:Assignment Section E Assertion-Reason Type Questions
Explore 9 Videos
- AMINES Book:AAKASH INSTITUTEChapter:AMINES Exercise:Assignment Section B (Objective Type Questions) one option is correct
Explore 22 Videos
- ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Book:AAKASH INSTITUTEChapter:ALDEHYDES, KETONES AND CARBOXYLIC ACIDS Exercise:Try Yourself
Explore 20 Videos
- BIOMOLECULESBook:AAKASH INSTITUTEChapter:BIOMOLECULESExercise:EXERCISE (ASSIGNMENT) SECTION - D Assertion - Reason Type Questions
Explore 5 Videos
Similar Questions
Knowledge Check
Activation of benzene by −NH2 group can be reduced by treating the compound with
Aacetic acidBacetyl chlorideCdilute HClDmethyl alcoholDuring electrophilic substitution reaction protection of −NH2 group)
AreductionBoxidationCalkylationDacylationStrong activating efferct of −NH2 group is reduced by using
ACH3COClBCH3ClCCH3OnaDCH3−CHO
In aniline , the −NH2 group
View SolutionIn aniline, the −NH2 group
View SolutionThe −NO2 group in an aromatic ring deactivates the ortho and para positions for an electrophilic attack. When −NO2 group is present at ortho or para positions of a leaving group (Nucleofuge) it activates the ring for nucleophilic attack. The reduction of −NO2 group by metal in acid causes its reduction to −NH2 group and then the ring becomes strongly activated for a electrophilic attack. The strong activation of −NH2 group is moderated by its acylation with CH3COCl to −NHAc group. Deacylation is carried out by hydrolysis with H3O+ or OH−. The ring alkylation by using RX/AlX3 is not possible in presence of −NO2 or −NH2 group but is possible in presence of −NHAc group.
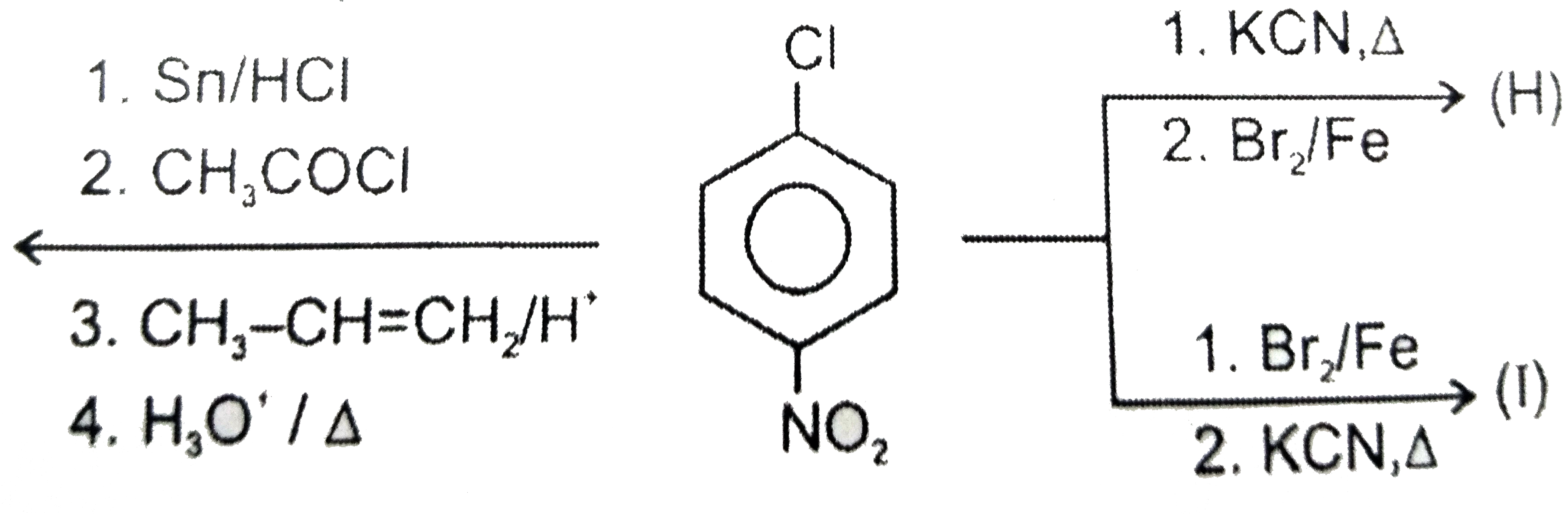
The product (I) is :
View SolutionThe −NO2 group in an aromatic ring deactivates the ortho and para positions for an electrophilic attack. When −NO2 group is present at ortho or para positions of a leaving group (Nucleofuge) it activates the ring for nucleophilic attack. The reduction of −NO2 group by metal in acid causes its reduction to −NH2 group and then the ring becomes strongly activated for a electrophilic attack. The strong activation of −NH2 group is moderated by its acylation with CH3COCl to −NHAc group. Deacylation is carried out by hydrolysis with H3O+ or OH−. The ring alkylation by using RX/AlX3 is not possible in presence of −NO2 or −NH2 group but is possible in presence of −NHAc group.
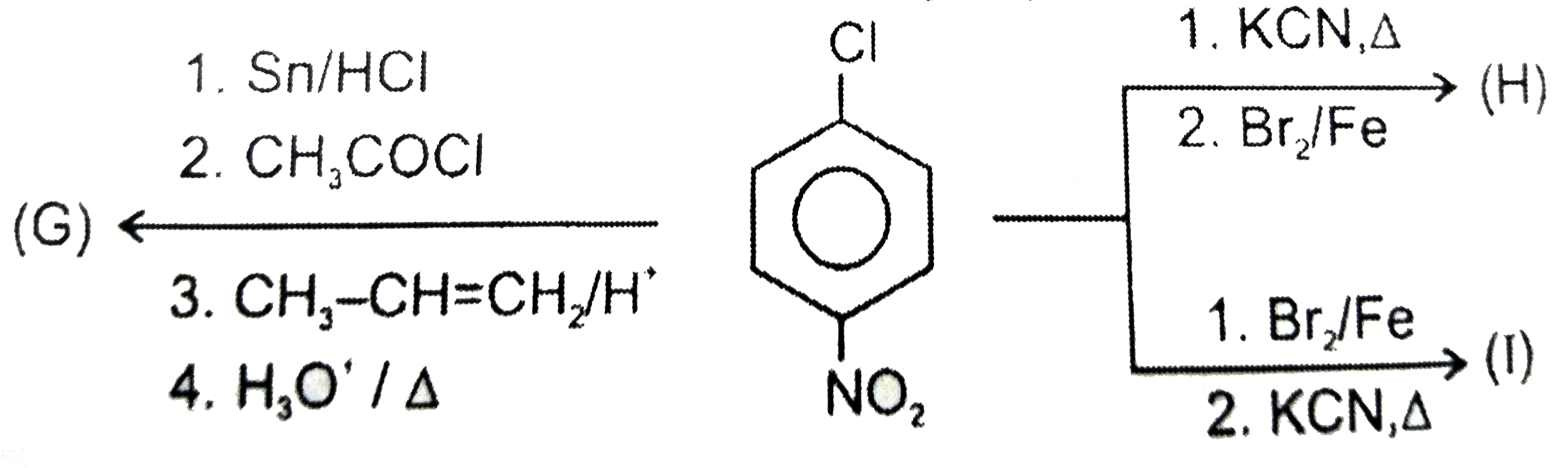
The product (G) is :
View SolutionThe −NO2 group in an aromatic ring deactivates the ortho and para positions for an electrophilic attack. When −NO2 group is present at ortho or para positions of a leaving group (Nucleofuge) it activates the ring for nucleophilic attack. The reduction of −NO2 group by metal in acid causes its reduction to −NH2 group and then the ring becomes strongly activated for a electrophilic attack. The strong activation of −NH2 group is moderated by its acylation with CH3COCl to −NHAc group. Deacylation is carried out by hydrolysis with H3O+ or OH−. The ring alkylation by using RX/AlX3 is not possible in presence of −NO2 or −NH2 group but is possible in presence of −NHAc group.
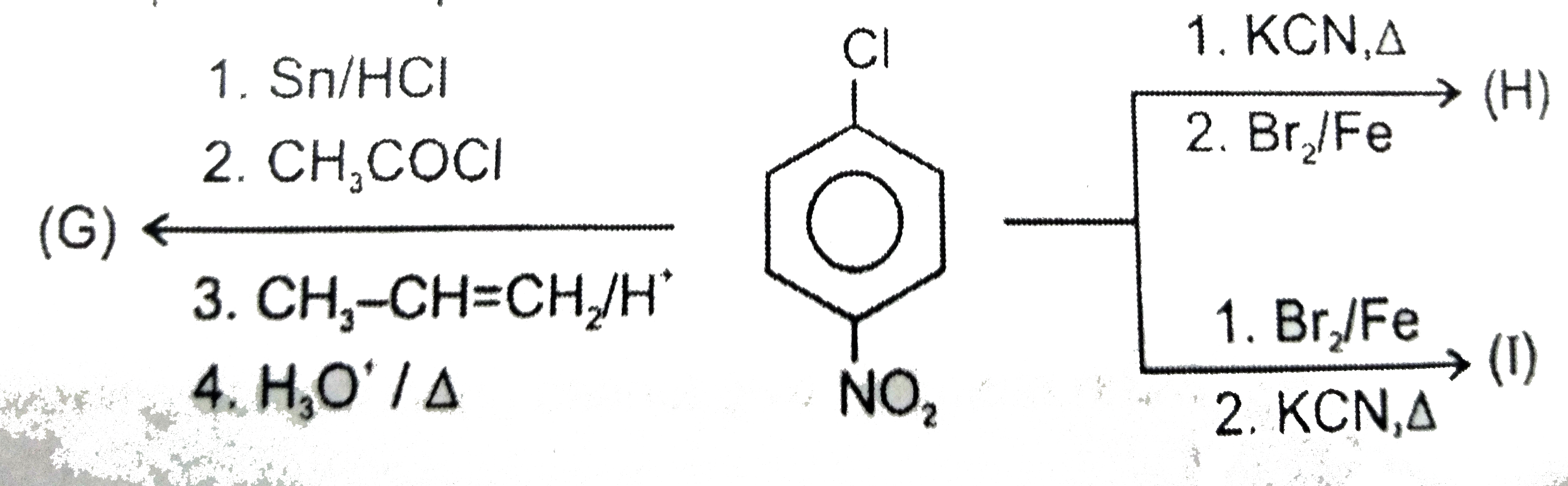
The product (H) is :
View Solution
