In cationic polymerizalion, the initiator is an electrophile that adds to the alkene, causing it to become a cation. The Initiator most often used in cationic polymerization is a lewis acid. Such as BF3 or AICI3 and in anionic polymerization, the iniliator is a nucleophile that reacts with the alkene to form a propagating site that is an anion.
Arrange the following group of monomers of decreasing ability to undergo cationic polymerization
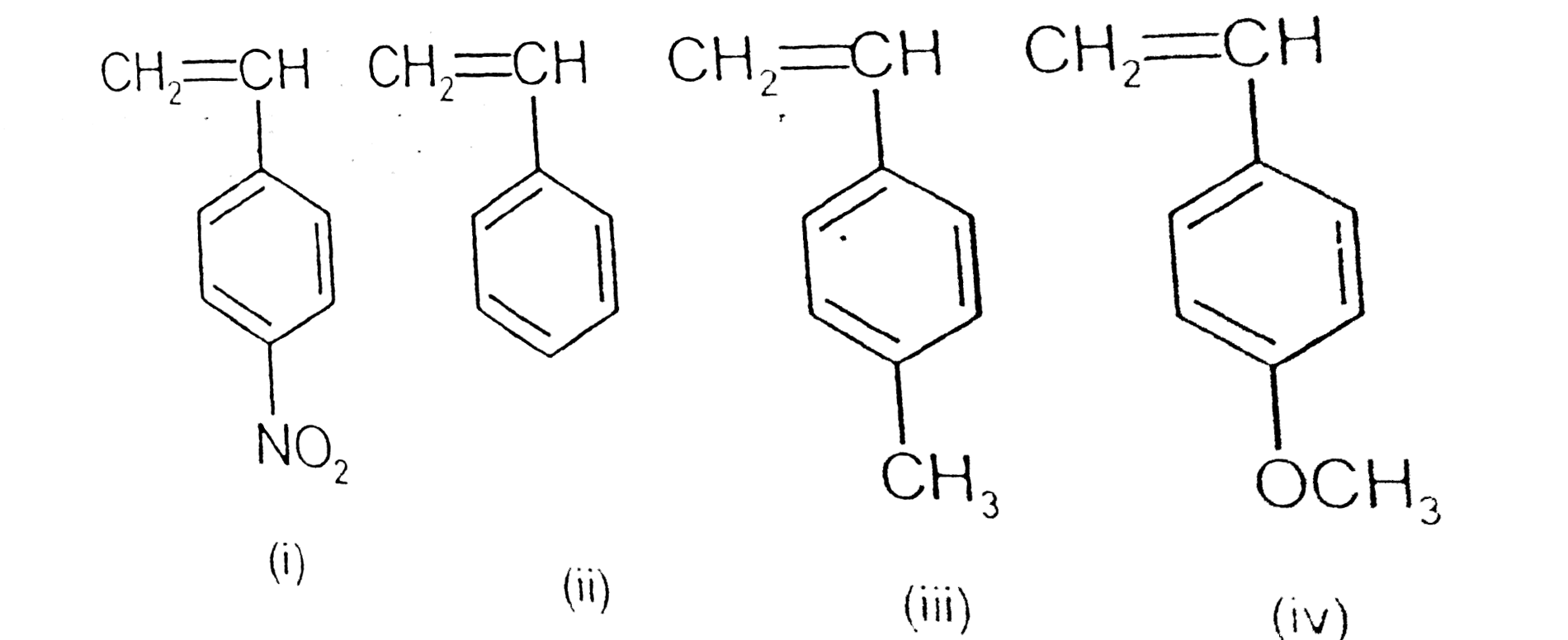
(iv)>(iii)>(ii)>(i)
(ii)>(iii)>(iv)>(i)
(iv)>(i)>(ii)>(iii)
(iv)>(ii)>(iii)>(i)
The correct Answer is:A
Step by step video & image solution for In cationic polymerizalion, the initiator is an electrophile that adds to the alkene, causing it to become a cation. The Initiator most often used in cationic polymerization is a lewis acid. Such as BF_(3) or AICI_(3) and in anionic polymerization, the iniliator is a nucleophile that reacts with the alkene to form a propagating site that is an anion. Arrange the following group of monomers of decreasing ability to undergo cationic polymerization by Chemistry experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- POLYMERSBOOK - AAKASH INSTITUTECHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section- E (Assertion-Reason Type Questions )
3 Videos
- POLYMERSBOOK - AAKASH INSTITUTECHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section-G (Integer Answer Type Questions )
4 Videos
- POLYMERSBOOK - AAKASH INSTITUTECHAPTER - POLYMERSEXERCISE - ASSIGNMENT Section -C (Objective type Questions (More one option is correct ))
8 Videos
- ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESBOOK - AAKASH INSTITUTECHAPTER - ORGANIC CHEMISTRY: SOME BASIC PRINCIPLE AND TECHNIQUESEXERCISE - TRY YOURSELF
27 Videos
- PRINCIPLES OF QUALITATIVE ANALYSISBOOK - AAKASH INSTITUTECHAPTER - PRINCIPLES OF QUALITATIVE ANALYSISEXERCISE - Assignment (SECTION H)
9 Videos
Similar Questions
Arrange the following groups of monomers in order of decreasing ability to undergo cationic polymerization :
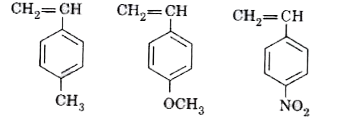
View SolutionIn cationic polymerizalion, the initiator is an electrophile that adds to the alkene, causing it to become a cation. The Initiator most often used in cationic polymerization is a lewis acid. Such as BF3 or AICI3 and in anionic polymerization, the iniliator is a nucleophile that reacts with the alkene to form a propagating site that is an anion.
What monomer and what type initiator would you use to synthesize following polymer ?
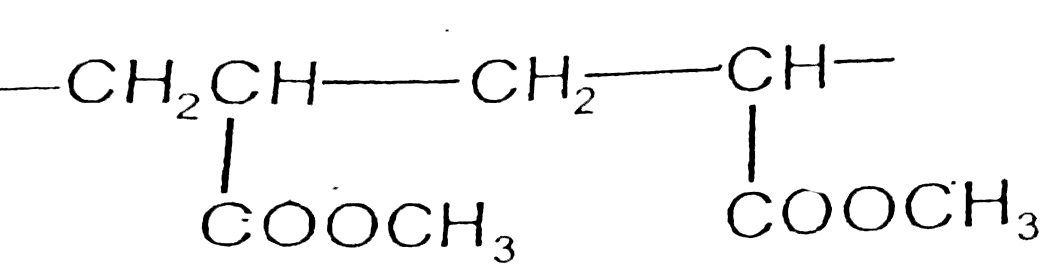
View Solution
Knowledge Check
In cationic polymerizalion, the initiator is an electrophile that adds to the alkene, causing it to become a cation. The Initiator most often used in cationic polymerization is a lewis acid. Such as BF3 or AICI3 and in anionic polymerization, the iniliator is a nucleophile that reacts with the alkene to form a propagating site that is an anion.
Which of the follówing represents anionic polymerisation?ABu−Li+CH2=CH∣CNBBF3+CH2=C(CH3)2C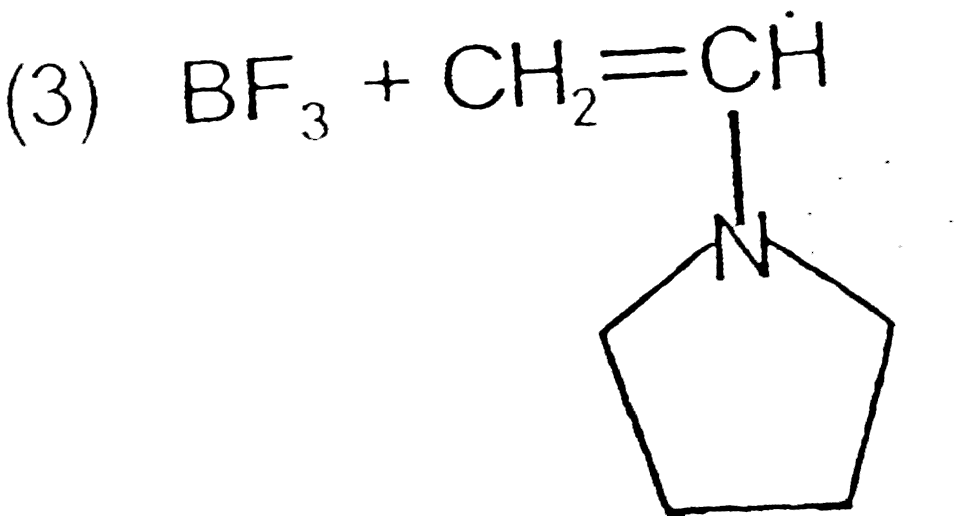 D
D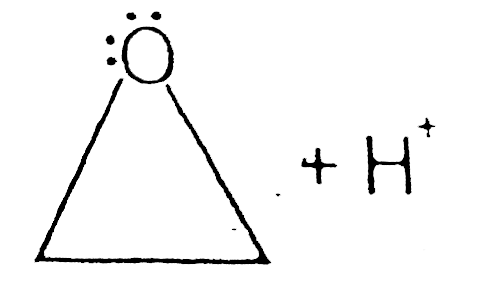
Anionic polymerization may be accomplished with
AstyreneBacrylonitrileCmethyl methyacrylateDAll of theseWhich of the following monomers would be most reactive towards cationic polymerization?
Aa. CH2=CHCH3Bb. CH2=CHClCc. CH2=CHC6H5Dd. CH2=CHCOOCH3
Distinguish between cations and anions.
View SolutionThe species which can best serve as an initiator for the cationic polymerization is:
View SolutionThe species which can best serve as an initiator for the cationic polymerization is:
View SolutionThe cations and anions are arranged n alternate form in:
View SolutionWhich of the following monomers can unergoes radical, cationic as well as anionic polymerisation with equal ease?
View Solution

