ΔU+ΔW=0is valid for
A
Adiabatic process
B
Isochoric process
C
Isobaric process
D
Isothermal process
Video Solution
Text Solution
The correct Answer is:A
|
Answer
Step by step video, text & image solution for DeltaU+DeltaW=0is valid for by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- THERMODYNAMICSBook:AAKASH INSTITUTEChapter:THERMODYNAMICSExercise:Assignment (Section-B) Objective Type Questions (one option is correct)
Explore 14 Videos
- THERMODYNAMICSBook:AAKASH INSTITUTEChapter:THERMODYNAMICSExercise:Assignment (Section-C) Objective Type Questions (More than one option are correct)
Explore 11 Videos
- THERMODYNAMICSBook:AAKASH INSTITUTEChapter:THERMODYNAMICSExercise:Try Youself
Explore 12 Videos
- THERMAL PROPERTIES OF MATTERBook:AAKASH INSTITUTEChapter:THERMAL PROPERTIES OF MATTERExercise:Assignment (Section-J) Akash Challengers Questions
Explore 7 Videos
- UNITS AND MEASUREMENTSBook:AAKASH INSTITUTEChapter:UNITS AND MEASUREMENTSExercise:ASSIGNMENT (SECTION - D)
Explore 15 Videos
Similar Questions
Knowledge Check
dU+dW=0 is valid for
Aadiabatic processBisothermal processCisobaric processDisochoric processGauss law is valid for
ASquare laminaBDiscCShellDNoneδU is equal to
AIsobaric workBAdiabatic workCIsothermal workDIsochoric work
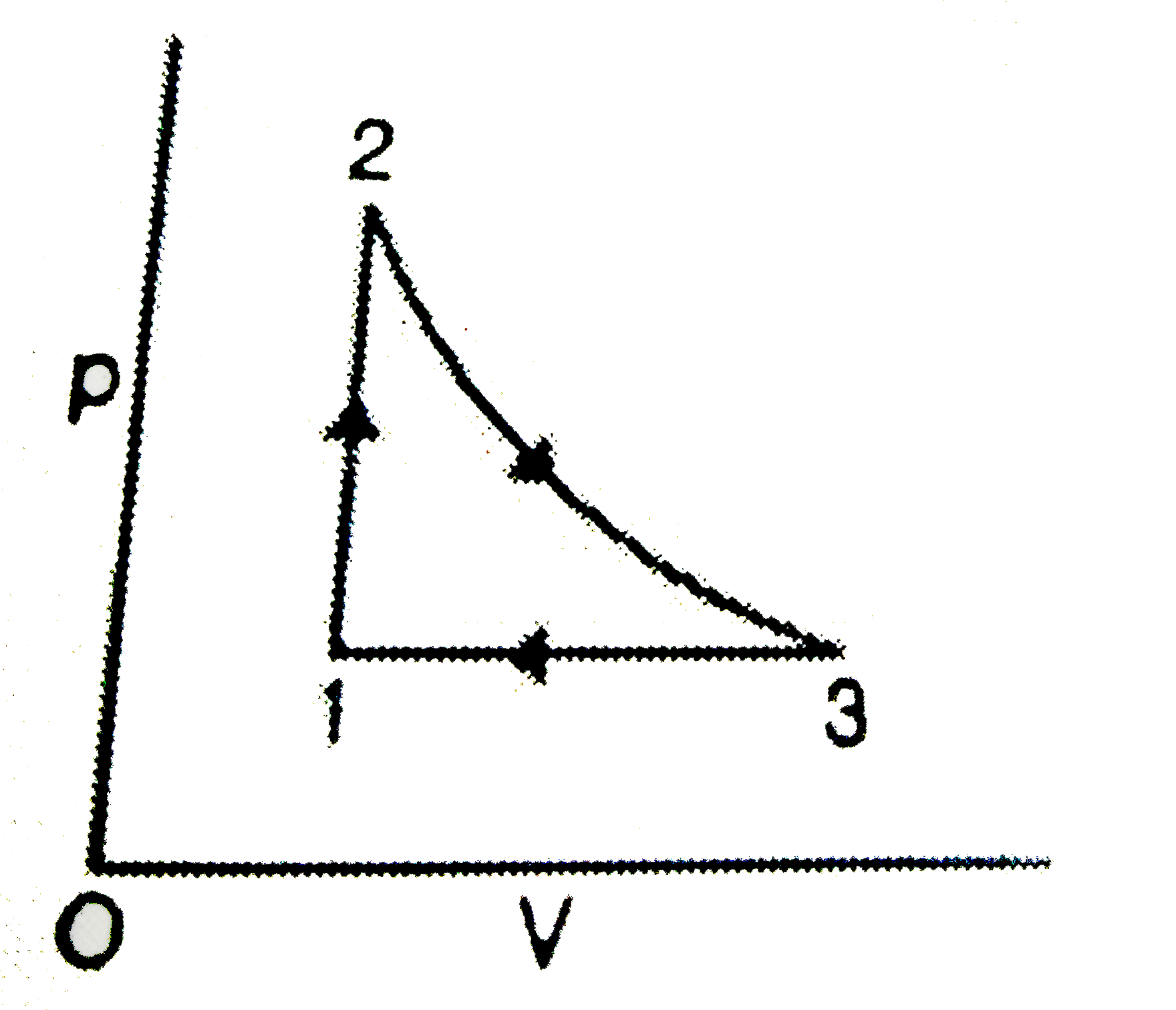
A cyclic process for an ideal monatomic gas (Cv=12.5Jmol−1K−1) is represented in the figure. The temperature at 1, 2 and 3 are 300K,600K and 455K, respectively. Compute the values of ΔQ,ΔU and ΔW for each of the process. The process from 2 to 3 is adiabatic.
View SolutionGauss's law is valid for
View SolutionCoulomb's law is valid for
View SolutionLaw of areas is valid for any
View SolutionBalmer formula is valid for
View Solution
