Consider a gas filled in a part of thermally insulated vessel with frictionless insulated piston (connected to a massless spring). Natural length of the spring equals length of the vessel. For 30 J of heat supplied, internal energy of gas increases by 25 J. find the number of atoms in one molecule.
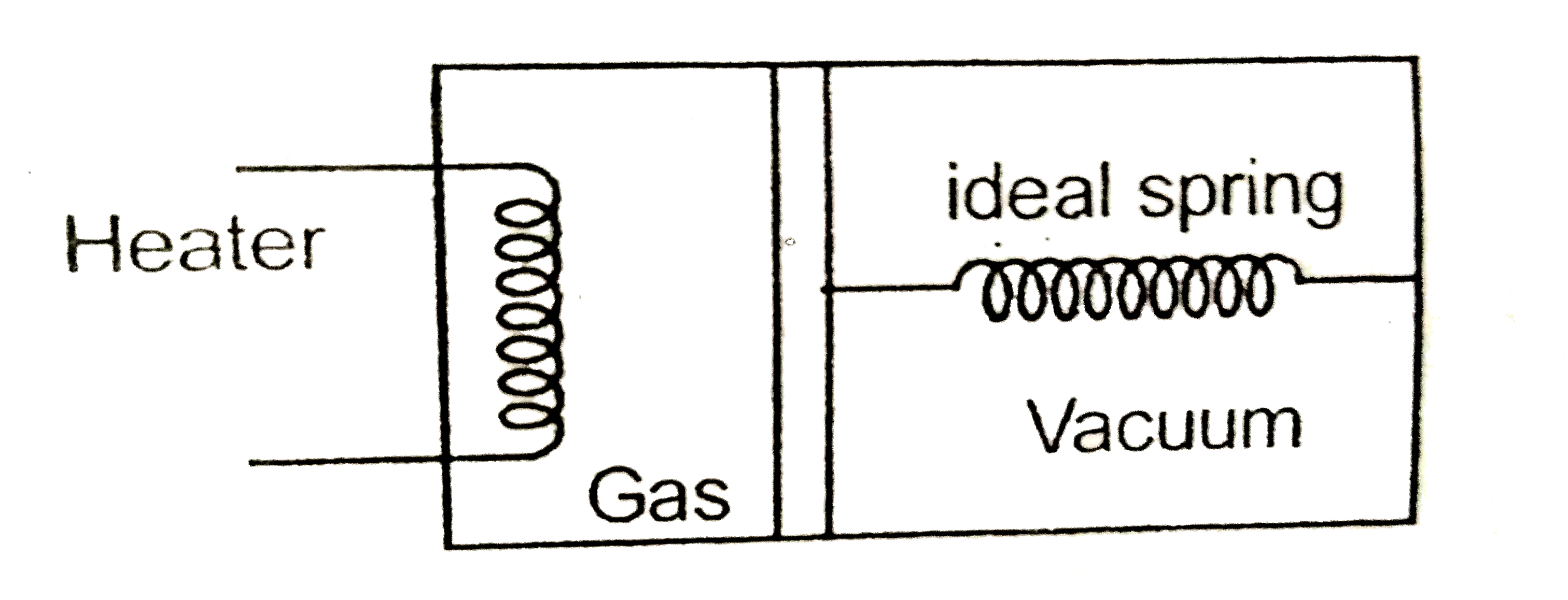
The correct Answer is:2
Step by step video solution for Consider a gas filled in a part of thermally insulated vessel with frictionless insulated piston (connected to a massless spring). Natural length of the spring equals length of the vessel. For 30 J of heat supplied, internal energy of gas increases by 25 J. find the number of atoms in one molecule. by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023टॉपर्स ने हल किए ये सवाल
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - TRY YOURSELF
11 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - EXERCISE
19 Videos
- THERMODYNAMICSBOOK - AAKASH INSTITUTECHAPTER - THERMODYNAMICSEXERCISE - Assignment (Section-I) Subjective Type
5 Videos
- THERMAL PROPERTIES OF MATTERBOOK - AAKASH INSTITUTECHAPTER - THERMAL PROPERTIES OF MATTEREXERCISE - Assignment (Section-J) Akash Challengers Questions
7 Videos
- UNITS AND MEASUREMENTSBOOK - AAKASH INSTITUTECHAPTER - UNITS AND MEASUREMENTSEXERCISE - ASSIGNMENT (SECTION - D)
15 Videos
समान प्रश्न
What are the conditions for thermodynamic equilibrium?
View SolutionTwo bodies A and B are said to be in thermal equilibrium with each other if they have same
View SolutionTwo moles of an ideal gas are contained in a vertical cylinder with a frictionless piston. The piston is slowly displaced so that expansionin gas is isothermal at temperature T0=400K. find the amount of work done in increasing the volume to 3 times.t ake atmospheric pressure =105N/m2
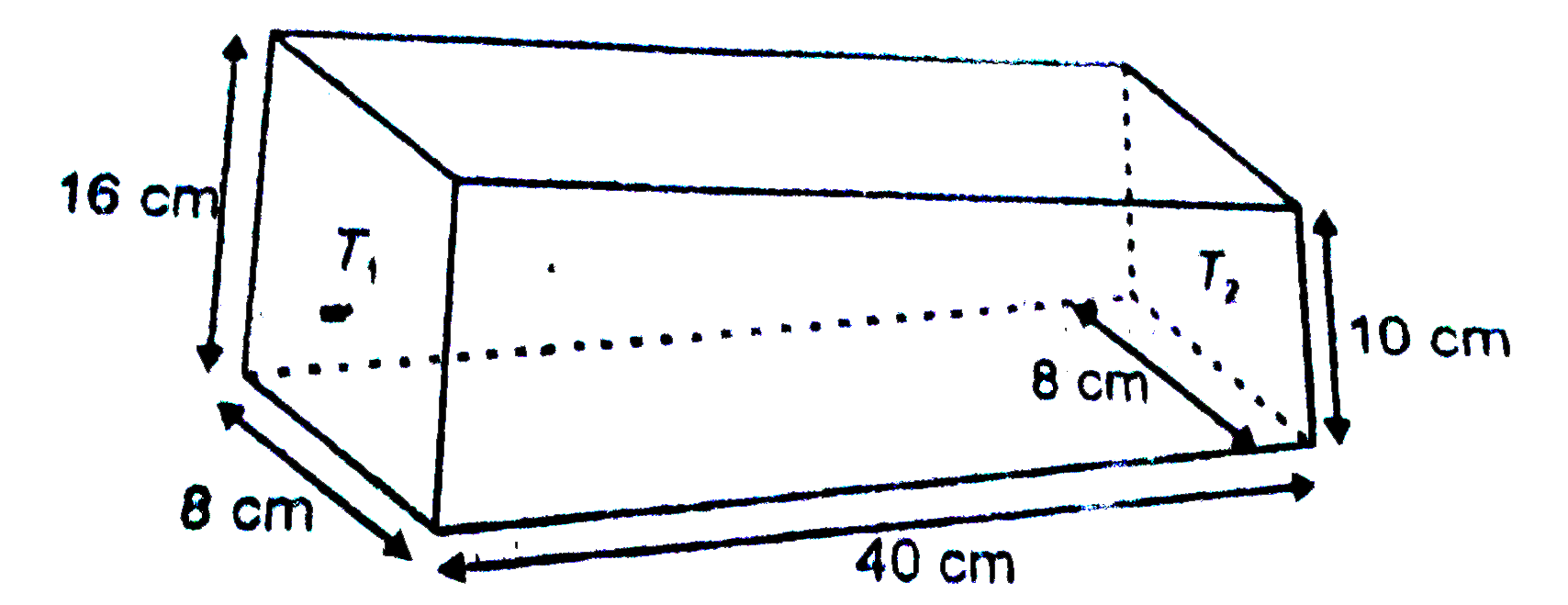
View SolutionA slab of cunductivity k is in the shape of trapezoid as shown I figure. If the two end faces be maintained at temperature T1=50∘CandT2=25∘C, then determine the thermal current through the slab by ignoring any heat loss through the lateral surfaces.
 .
.
View SolutionA : PARSEC and light year, both measure time
R : Both have dimension of time.
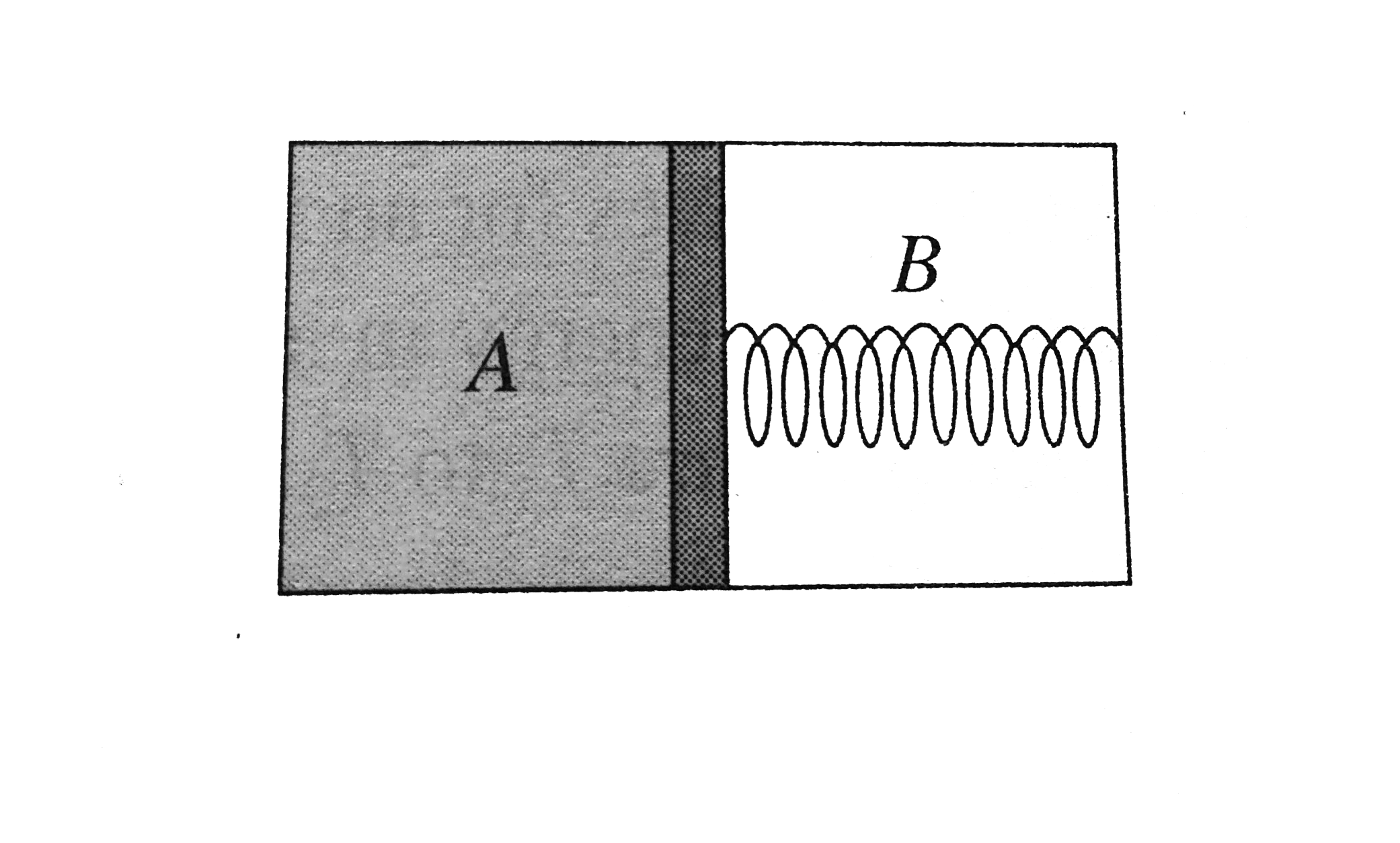
View SolutionA thermally insulated chamber of volume 2V0 is divided by a frictionless piston of area S into two equal part A and B. Part A has an ideal gas at pressrue P0 and temperature T0 and part B is vacuum. A massless spring of force constant K is connected with the piston and the wall of the container as shown. Initially the spring is unstretched. The gas inside chamber A is allowed to expand. Let in equilibrium the spring be compressed by x0. Then
View SolutionIn the arrangement shown in the fig the cylinder is insulating one. Both sides same diatomic gas is trapped by two insulting massless pistos with the help of an ideal spring. The natural length of the spring is equal to the length of the cylinder. initial state of the gases are as shown in the figure. What is the value of energy stored in the spring ?
View SolutionTwo identical vessels A & B contain equal amount of ideal monoatomic gas. The piston of A is fixed but that of B is free. Same amount of heat is absorbe3d by A & B 's internal energy increases by 100 J the change in internal energy of A is
View SolutionA thermally insulated vessel is divided in to two parts by a heat insulating piston which can move in the vessel without the friction. The left part of the vessel contains one mole of an ideal monatomic gas, & the right part is empty. The piston is connected to the right wall of the vessel through a spring whose length in free state is equal to the length of the vessel as shown in the figure. Determine the heat capacity C of the system, neglecting the heat capacity of the vessel, piston and spring.

View SolutionAn ideal gas is enclosed in a thermally insulated container. Container is covered by an insulating piston which is connected with spring. Small displacement is given to piston as shown in figure. Which of the following statements is correct? Neglect friction everywhere.
View SolutionA thermally insulated vessel containing monatomic gas is moving with a speed of 30 m/s. If the vessel suddenly stops, the increase in gas temperature is (Molar mass of gas = 83 g/mol and R = 8.3J/K mol)
View SolutionA thermally insulated vessel containing monatomic gas is moving with a speed of 30 m/s. If the vessel suddenly stops, the increase in gas temperature is (Molar mass of gas = 83 g/mol and R = 8.3J/K mol)
View SolutionAn insulated cylinderical vessel filled with an insulated piston of negligible weight and negligible thickness at the mid point of the vessel . The cylinder contains a gas at 0^@C .When the gas is heated to 100^@C , the piston moves through a length of 5 cm . Length of the cylindrical vessel in cm is
View SolutionA cylindrical vessel made of thermally insulating material is divided into two equal parts by an insulating and. Movable piston. Both parts contain ideal monoatomic gas . The gas in the left part is supplied heat such that the volume of right part becomes one-eight of its initial volume . Work done by the gas in the right part is
View SolutionAn ideal gas is kept in a closed, rigid and heat insulating vessel. A coil of resistance 100 ohm, carrying a current of 1 ampere, is supplying heat to the gas for 5 minutes. What will be the change of internal energy of the gas?
View Solution

