H3PO3 can be represented by the structures 1 and 2 shown below . Can these two structrue be
taken as the canonical forms of the resonance hybrid of H3PO3? If not ,given reason for the same
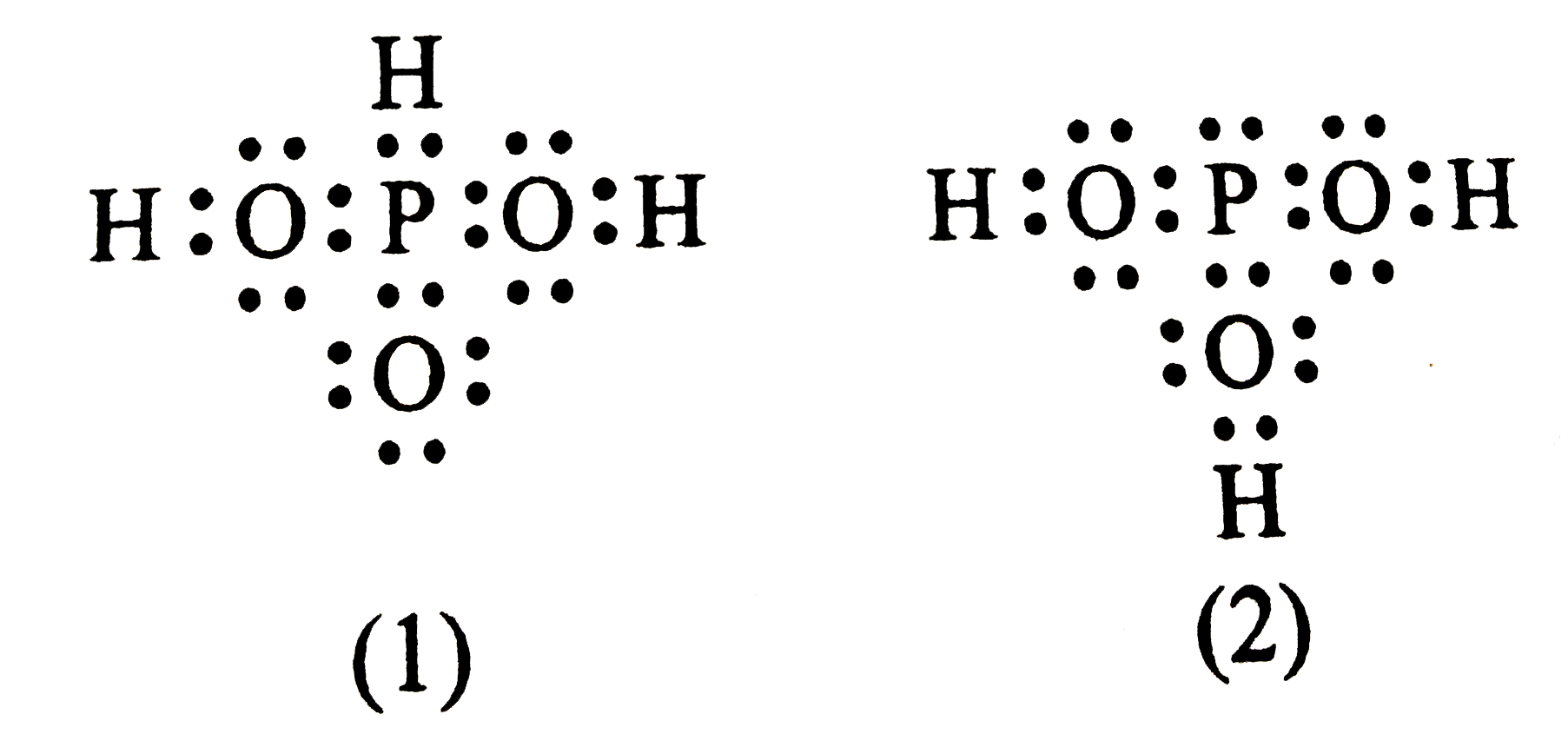
Video Solution
Text Solution
No,these cannot be taken as cononical forms because the positions of atoms have been changed .
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -I)
Explore 22 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -II)
Explore 1 Video
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:CONCEPTUAL QUESTIONS
Explore 42 Videos
- APPENDIXBook:PRADEEPChapter:APPENDIXExercise:MODEL TEST PAPER <br> (Section C )
Explore 9 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:PRADEEPChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
Explore 12 Videos
Similar Questions
H3PO3 can be represented by structure (a) and (b) shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3 ?If not, give reasons for the same.
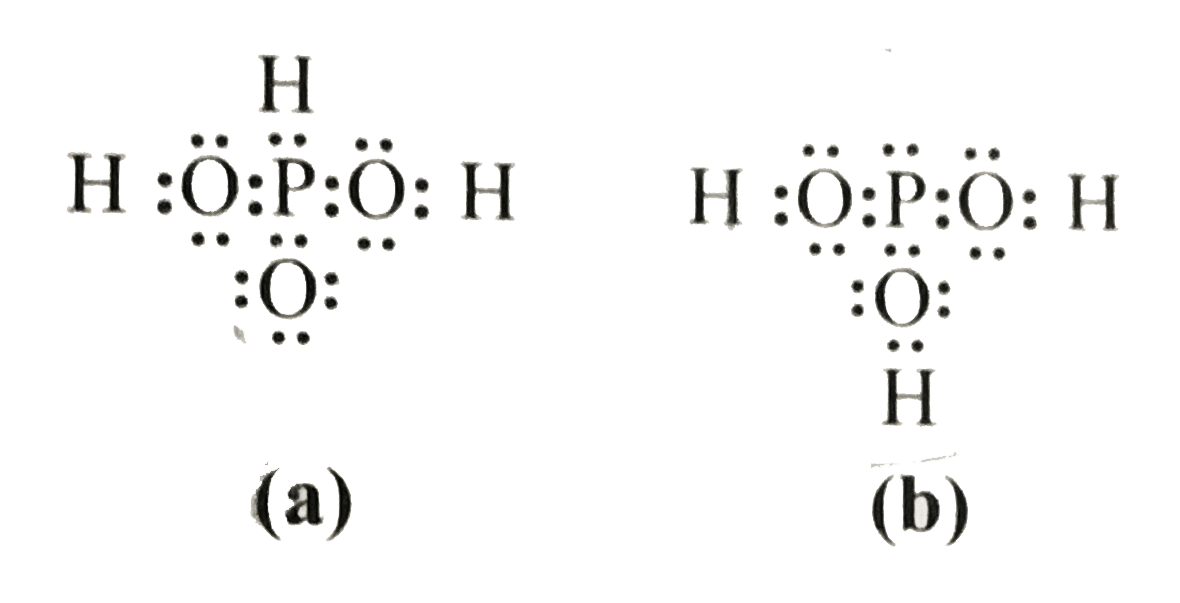
View SolutionH3PO3 can be represented by structures I and II shown below. Can these two structures be taken as the canonical forms of the resonance hybride representing H3PO3? If not, give resonance hybrid representing H3PO3? If not, give reasons for the same.
View Solution
Knowledge Check
H3PO3 is
AA tribasicBA dibasicCA monobasicDNeutralH3PO3 is
AA tribasic acidBA dibasic acidCNeutralDA monobasic acidIn H3PO3 molecule
AP-atom is surrounded by three -OH groupsBP-atom is tetrahedrally surrounded b two -OH groups, one oxygen atom and onee hydrogen atomCP-atom is surounded by four -OH groupsDP-atom is surrounded by two -H atoms
