Write Lewis structure of the following compounds and show format charge on each atom.
HNO3,NO2,H2SO4
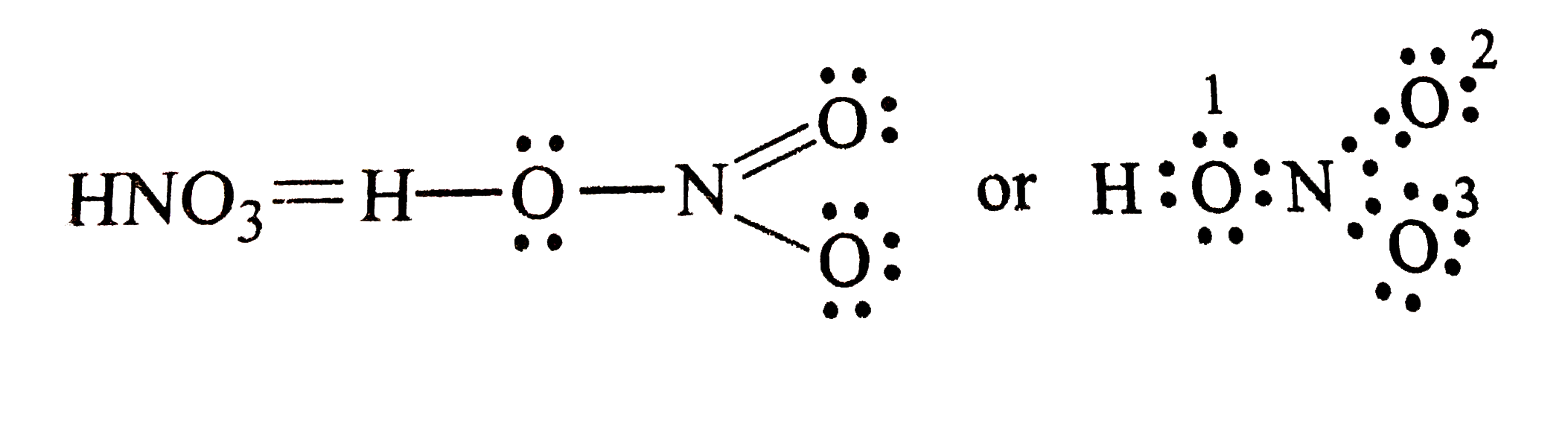
Formal charge = V−L−12S
Formal charge on H = 1−0−12XX2=0
Formal charge on O(1)=6−4−12(4)=0
formal charge on (1) N=5−0−12(8)=1
Formal charge on O(2)=6−4−12(4)=0
Formal charge on O(3)=6−6−12(2)=−
Hence , we write the structure of HNO3 with write structrue of HNO3 formal charges as 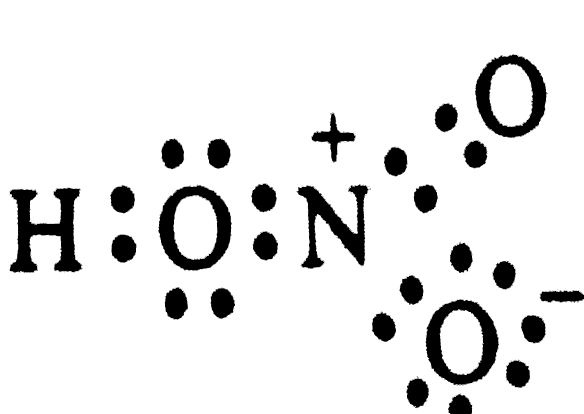
Similary, we have 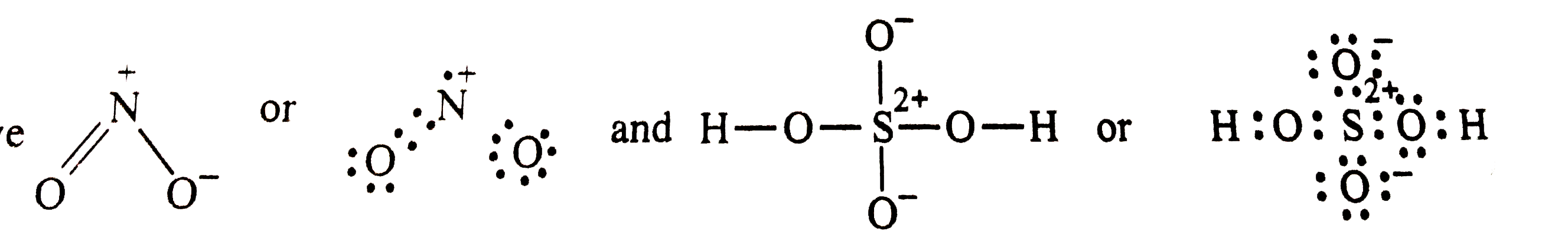
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - PRADEEPCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT EXAMPLAR PROBLEMS (MATCHING TYPE QUESTIONS)
5 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - PRADEEPCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT EXAMPLAR PROBLEMS (ASSERTION AND REASON TYPE QUESTIONS)
3 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBOOK - PRADEEPCHAPTER - CHEMICAL BONDING AND MOLECULAR STRUCTUREEXERCISE - NCERT EXAMPLAR PROBLEMS (MULTIPLE CHOICE QUESTIONS -1)
7 Videos
- APPENDIXBOOK - PRADEEPCHAPTER - APPENDIXEXERCISE - MODEL TEST PAPER <br> (Section C )
9 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES BOOK - PRADEEPCHAPTER - CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES EXERCISE - Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
12 Videos
Similar Questions
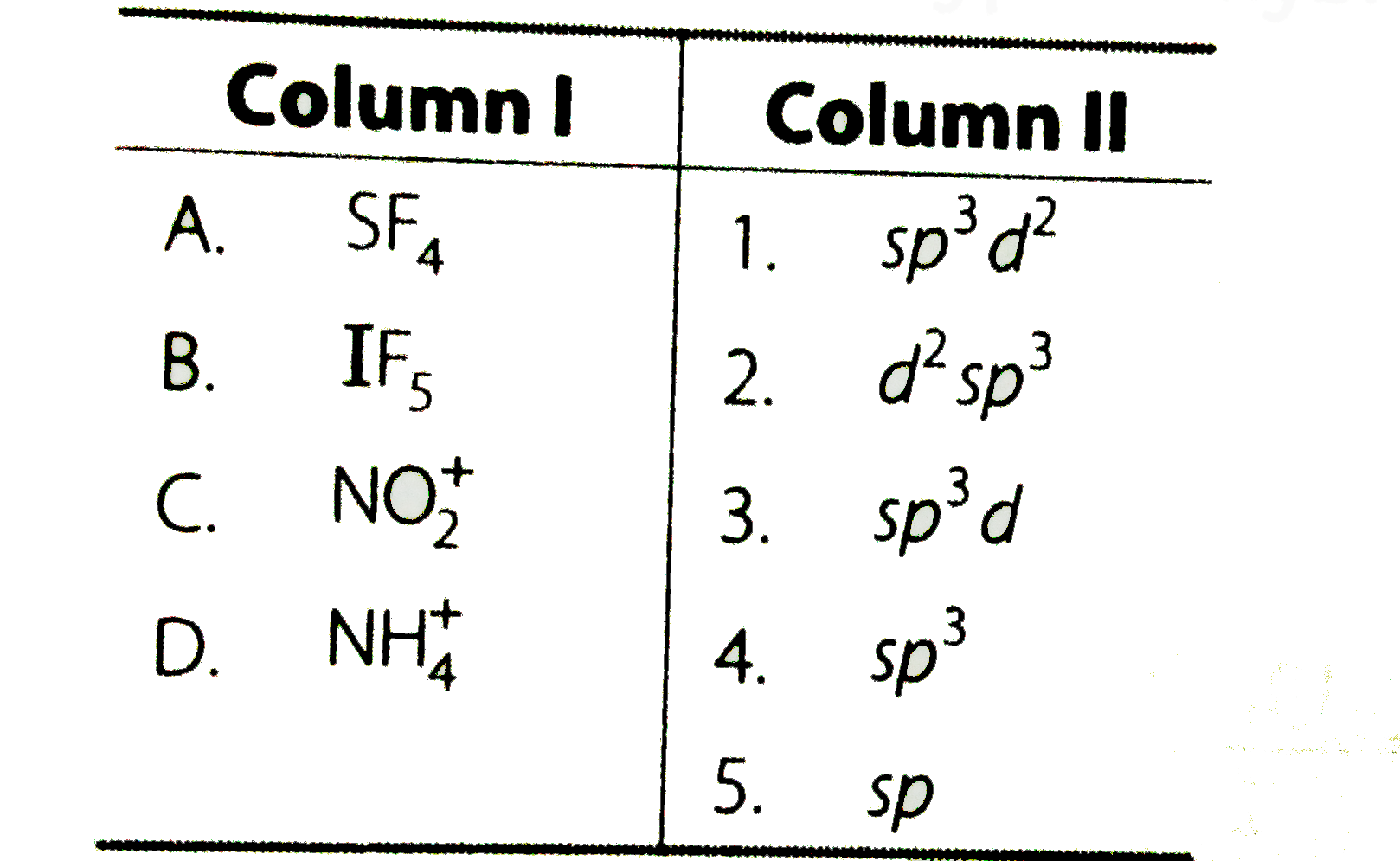
Match the species in Column I with the type of hybrid orbitals in Column II.
View SolutionAssertion (A): Sodium chloride formed by the action of chlorine gas on sodium metal is a stable compound.
Reason: (R) This is because sodium and chloride ions acquire octet in sodium chloride formation.
View SolutionWhich of the following attain the linear structure ?
View SolutionCalcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
What mass of CaCO3 is required to react completely with 25mL of 0.75MHCl?
View SolutionIn each of the following questions a Statement of Assertion (A ) is given followed by a corresponding statement of Reason (R ) Just below it . Of the statement mark the correct answer as
Assertion .Helium and beryllim have similar outer electronic configuration of the type ns2
Reason . both are chemcially inert .
View SolutionWrite the resonance structures for SO3,NO2, and NOө3.
View SolutionAssertion : A mixture of HNO_(3) and H_(2)SO_(4) is used for the nitration of benzene. Reason : H_(2)SO_(4) works as an acid HNO_(3) as a base to produce NO_(2)^(+) ioni.
View SolutionWrite Lewis structure of the following compounds and show format charge on each atom.
HNO3,NO2,H2SO4
View SolutionSO3,NO2 तथा NO−3 की अनुनादी संरचनाएँ लिखिए ।
View Solutionउपसह -संयोजक आबन्ध से क्या अभिप्राय है तथा ये किस प्रकार निर्मित होते है ? निम्नलिखित अणुओं की लुईस संरचनाएँ लिखिए -
O3,SO2,SO3,H2SO4 तथा NH+4
View SolutionSO3,NO2 तथा NO−3 की अनुनाद-संरचनाऍं लिखिए।
View SolutionBalance the following redox reaction (i) SnO_(2)+c toSn+CO (ii) Fe_(3)O_(4)+c to Fe +CO (iii) I_(2)+HNO_(3) to H_(2)SO_(4) to Fe_(2)(SO_(4))_(3)+NO+H_(2)O (v)Fe+HNO_(3) to Fe(NO_(3))_(2)+NH_(4)NO_(3)+H_(2)O (vi) Sb+HNO_(3) to H_(3)SbO_(4)+NO_(2)+H_(2)O (vii) Hg+HNO_(3) to Hg_(2)(NO_(3))_(2)+NO+H_(2)O
View SolutionBalance the following redox reaction (i) SnO_(2)+c toSn+CO (ii) Fe_(3)O_(4)+c to Fe +CO (iii) I_(2)+HNO_(3) to H_(2)SO_(4) to Fe_(2)(SO_(4))_(3)+NO+H_(2)O (v)Fe+HNO_(3) to Fe(NO_(3))_(2)+NH_(4)NO_(3)+H_(2)O (vi) Sb+HNO_(3) to H_(3)SbO_(4)+NO_(2)+H_(2)O (vii) Hg+HNO_(3) to Hg_(2)(NO_(3))_(2)+NO+H_(2)O
View SolutionWrite the resonance structures for SO3,NO2,NO−3.
View SolutionBalance the following equation using oxidation number method. S + HNO_(3) rarr H_(2)SO_(4) + NO_(2) + H_(2)O
View Solution

