Explain, why o-hydroxybenzaldehyde is a liquid at room temperature while p-hydroxybenzaldehyde is a high melting solid?
Video Solution
Text Solution
In o-hydroxybenzalde , there is intrmolecular hydrogen bonding which further prevents association
of the molecular . In p-hydroxy benzaldehyde, there is intermolecular hydrogen bonding and hence , there
is association among the moleculas .
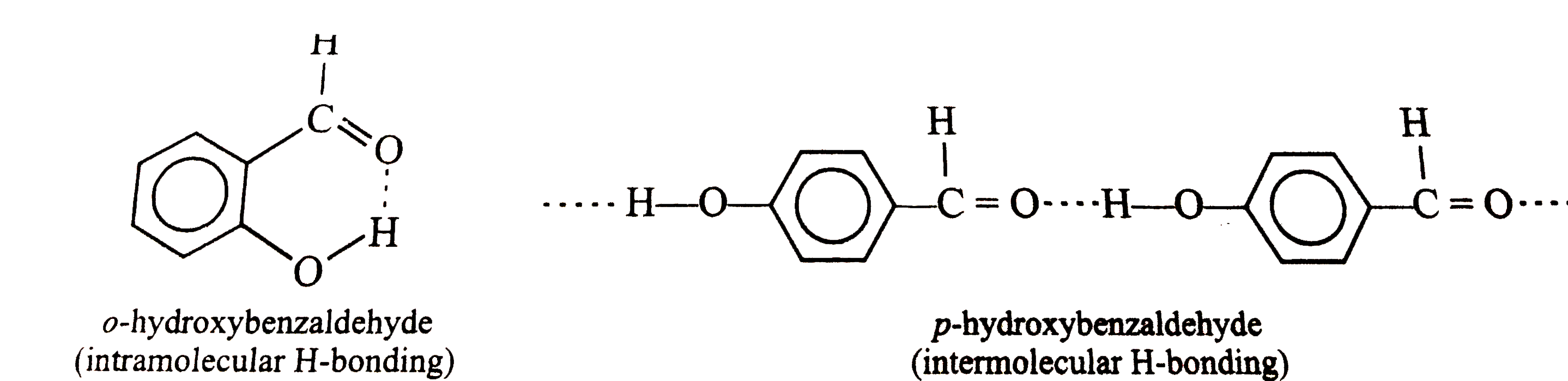
As a result , o-hydroxybenzaldehyde is a liquid whereas p-hydroxybenzaldehyde is a solid .
Similarly, in case of nitrophenol , we have
 .
.
|
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 3 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (I. MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER))
Explore 119 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (Questions)
Explore 1 Video
- APPENDIXBook:PRADEEPChapter:APPENDIXExercise:MODEL TEST PAPER <br> (Section C )
Explore 9 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:PRADEEPChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
Explore 12 Videos
Similar Questions
Knowledge Check
Which is solid at room temperature
ACOBCO2CSiO2DOF2Hydrocarbon that is liquid at room temperature is:
A(a) EthaneB(b) PropaneC(c) ButaneD(d) PentaneIodine is a solid at room temperature because
AIt has a low value of electronegativityBIt has a large sizeCThe magnitude of van der Waals forces is very large due to larger molecular sizeDIt has d-orbitals.
Explain, why o-hydroxybenzaldehyde is a liquid at room temperature while p-hydroxybenzaldehyde is a high melting solid?
View SolutionExplain why is o-hydroxybenzaldehyde a liquis at room temperaturre while p-hydroxybenzaldehyde is a high melting solid.
View Solutiono-Hydroxy benzaldehyde is more soluble in water than p-hybroxy benzaldehde
(b) o-Hydroxy benzaldehyde is liquid at room temperature while p-hydroxy benzaldehyde is high melting solid .
View SolutionExplain ,Who o-hydroxy bezaldehyde is a high melting
View SolutionThe halogen which is liquid at room temperature is ……………
View Solution
