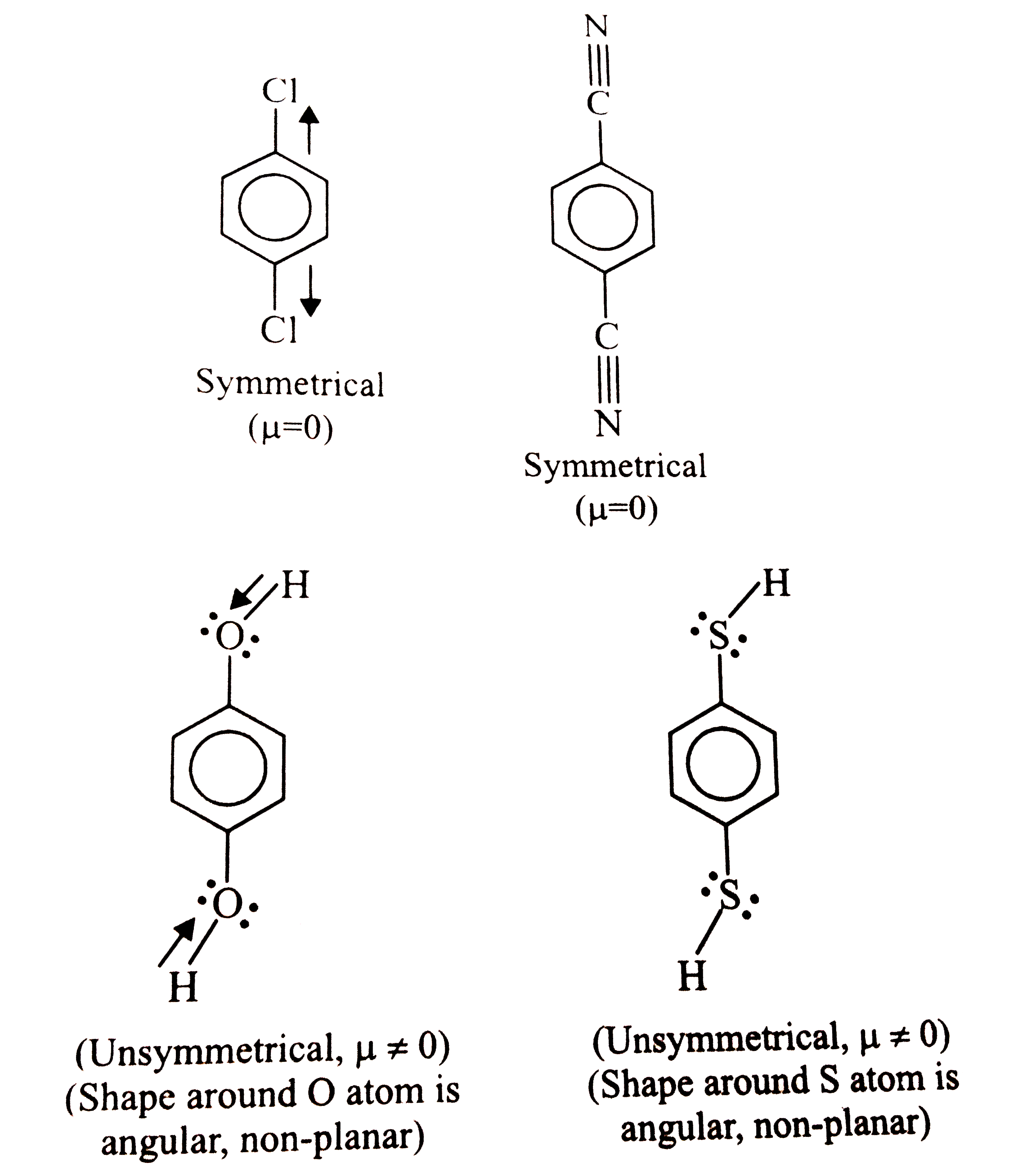
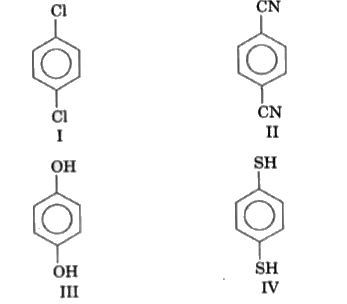
For which of the following molecules, significant
μ≠o ?
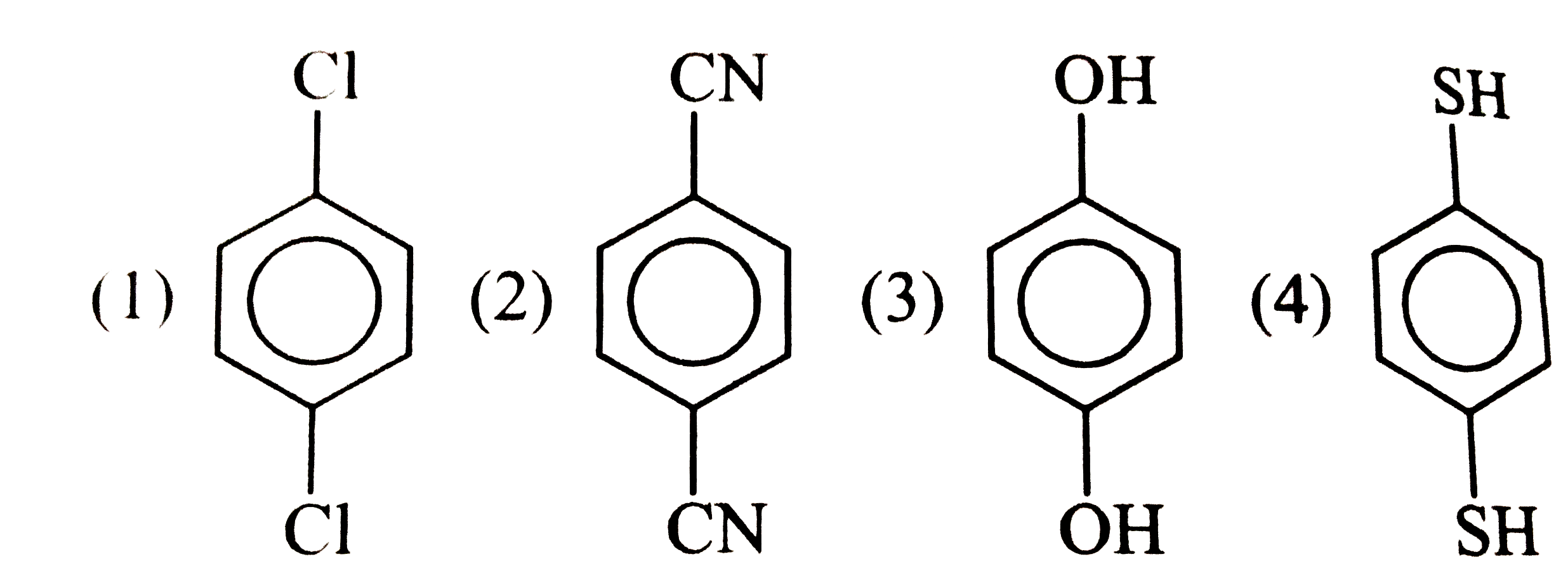
3 and 4
Only 1
1 and 2
4 only
The correct Answer is:A
Topper's Solved these Questions
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (II. MULTIPLE CHOICE QUESTINS (WITH ONE OR MORE THAN ONE CORRECT ANSWER))
Explore 13 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:COMPETITION FOCUS JEE (Main and Advanced)/ MEDICAL ENTRANCE SPECIAL (III. MULTIPLE CHOICE QUESTIONS (Based on the given Passage/Comprehension))
Explore 11 Videos
- CHEMICAL BONDING AND MOLECULAR STRUCTUREBook:PRADEEPChapter:CHEMICAL BONDING AND MOLECULAR STRUCTUREExercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 3 Videos
- APPENDIXBook:PRADEEPChapter:APPENDIXExercise:MODEL TEST PAPER <br> (Section C )
Explore 9 Videos
- CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Book:PRADEEPChapter:CLASSIFICATION OF ELEMENTS AND PERIODICITY IN PROPERTIES Exercise:Competition Focus (Jee Main and Advanced / Medical Entrance ) ( Assertion - Reason Type Question ) (Type II)
Explore 12 Videos
Similar Questions
Which of the following statements are not correct ?
View SolutionThe study of diple moment of a molecule is useful to explain the shape of a molecule and also to predict a The net dipole moment of a polyatomic molecule is the resltant of the different bond moments present in that molecule . The values are generalluy expressed in Debye or in the S.I. units in terms of Coulomb meter (C m) 1 Debye is equivalent to
View SolutionThe dipole momnet of KCI is 3.336×10−29Cm which indicates that it is a highly polar molecules. The inter atomic distance between K⊕ and CIΘ in this molecules is 2.6×10−10 m Calculate the dipole moment of KCI molecule if there were opposite charges of one fundamental unit located at each nucleus Calculate the ionic character percentage of KCI .
View SolutionCalcium carbonate reacts with aqueous HCl to give CaCl2 and CO2 according to the reaction:
CaCO3(s)+2HCl(aq)→CaCl2(aq)+CO2(g)+H2O(l)
What mass of CaCO3 is required to react completely with 25mL of 0.75MHCl?
View SolutionIn each of the following questions a Statement of Assertion (A ) is given followed by a corresponding statement of Reason (R ) Just below it . Of the statement mark the correct answer as
Assertion .Helium and beryllim have similar outer electronic configuration of the type ns2
Reason . both are chemcially inert .
View SolutionFor which of the following molecule significant mu ne0 ?
View SolutionWhich of the following molecules significant mu !=0 ?
View SolutionFor which of the following molecule significant mune0 ?
View SolutionIn which of the following molecules μ(oberved) is found to be greater than μ (theoretical):
View SolutionFor which of the following molecule significant mu ne 0 ?
View SolutionFor which of the following molecule significant mu ne 0 ?
View SolutionFor which of the following molecule significant mu ne 0 ?
View SolutionFor which of the following molecule significant mu ne 0 ?
View SolutionFor which of the following molecule significant mune0 ?
View SolutionFor which of the following molecules to the greatest extent mu ne 0 Will happen
View Solutionनिम्न में से किस अणु के लिए 2μ≠0 सार्थक होगा?
View Solution