In the titration of a weak acid aginst a strong base, at the half-equivalence point (half. Neutralisation )
pH=12pKa
pH=pKa
pH=2pKa
None of these
The correct Answer is:B
Topper's Solved these Questions
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Test Your Grip (II. Fill in the blanks)
Explore 20 Videos
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Conceptual Questions (I. Storng and weak electrolytes, ionic equilibrium and Ostwalds dilution law)
Explore 1 Video
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Advanced Problems (For Competitions)
Explore 19 Videos
- ENVIRONMENTAL CHEMISTRYBook:PRADEEPChapter:ENVIRONMENTAL CHEMISTRYExercise:COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II
Explore 6 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
Similar Questions
Knowledge Check
Assertion : In a titration of weak monoprotic acid with strong base, the pH at the half equivalent point is pKa.
Reason : At half equivalence point, it will form acidic buffer at its maximum capacity where [acid]=[salt].AIf both the statements are true and statement -2 is the correct explanation of statement-IBIf both the statements are true but statement-2 is not the correct explanation of statement-ICIf statement-I is true and statement-2 is falseDIf statement-I is false and statement-2 is true.Assertion : In a titration of weak monoprotic acid with strong base, the pH at the half equivalent point is pKa.
Reason : At half equivalence point, it will form acidic buffer at its maximum capacity where [acid]=[salt].AIf both assertion and reason are true and the reason is the correct explantion of the assertion.BIf both assertion and reason are true but reason is not the correct explanation of the assertion.CIf assertion is true but reason is false.DIf assertion is false but reason is true.Which of the following pH curve represent the titration of weak acid and strong base (dotted line show equivalence point)?
A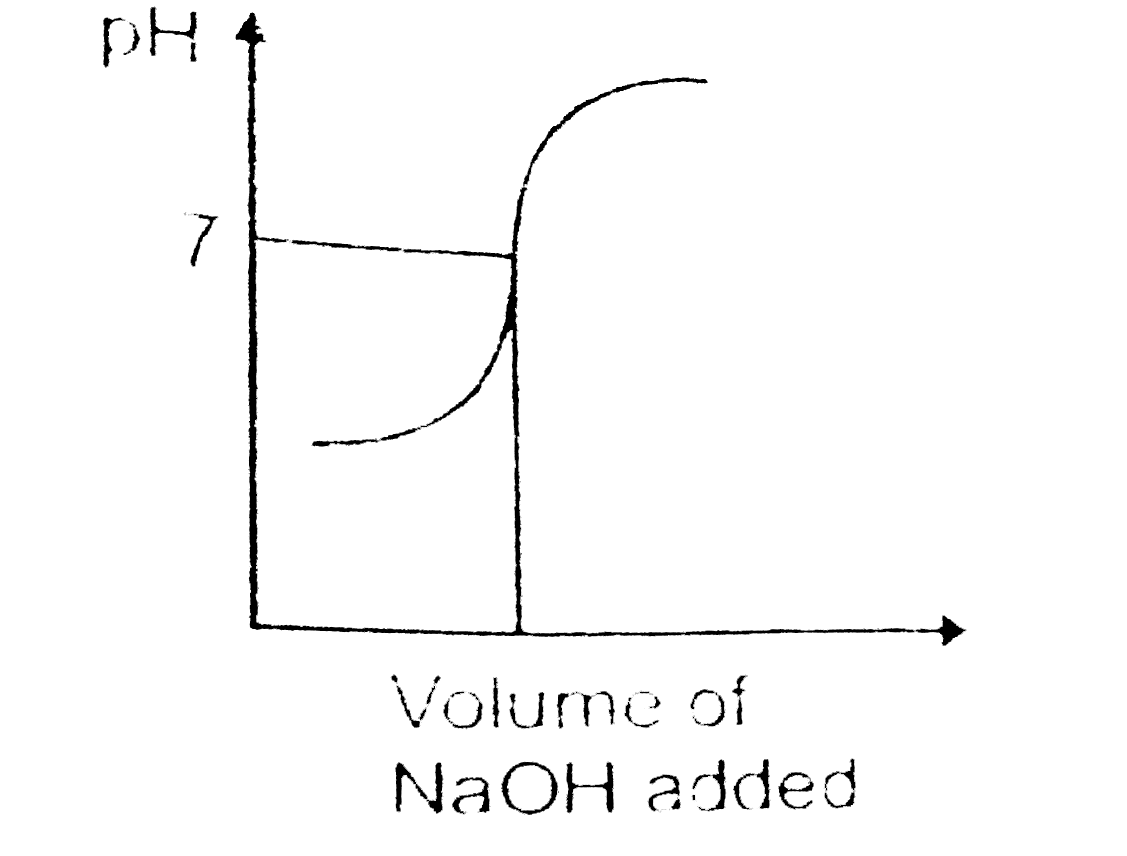 B
B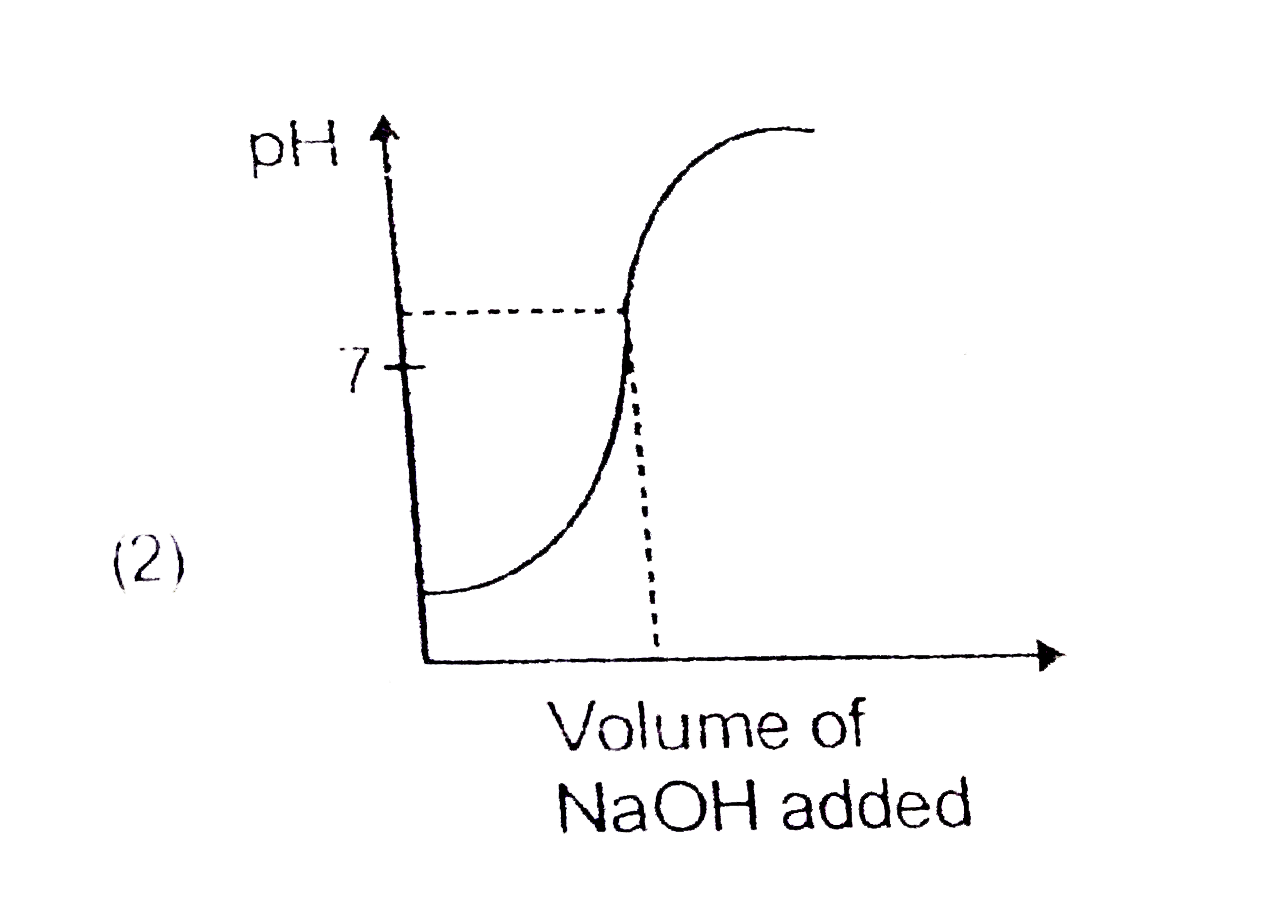 C
C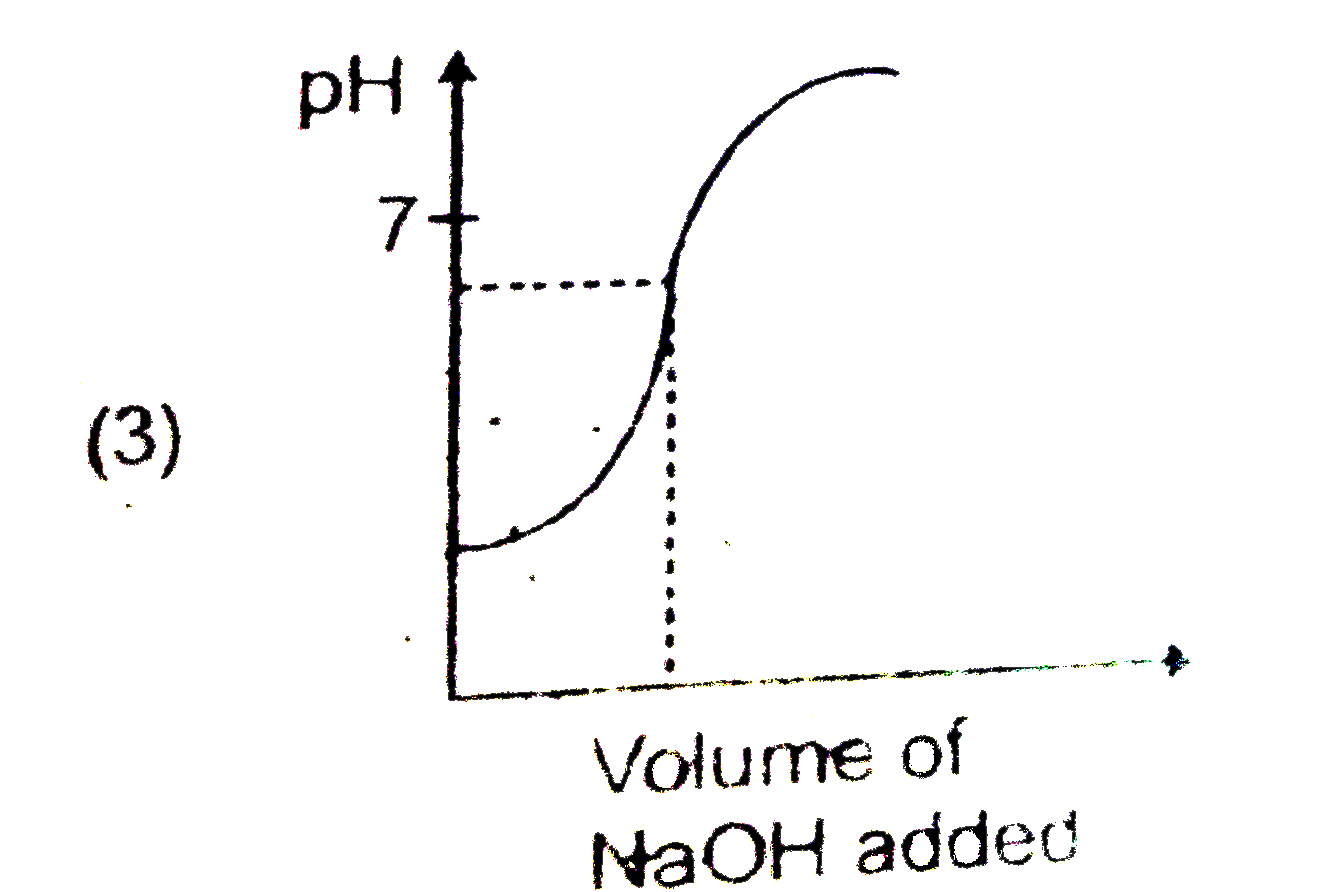 D
D
During the titration of a weak diprotic acid (H2A) against a strong base (NaOH), the pH of the solution half-way to the first equivalent point and that at the first equivalent point are given respectively by:
View SolutionAt half neutralisation of weal acid with a strong base,what is the relationship between pH and dissocaition constant (Ka) of weak acid ?
View Solution(A) In general phenolphthalein is used as an indicator for the titration of weak acid (CH3COOH) and strong base (NaOH).
(R) At equivalence point solution is basic.
View SolutionA strong acid is titrated with weak base. At equivalence point, pH will be :
View SolutionHeat of neutralisation of weak acid and strong base is less than the heat of neutralisation of strong acid and strong base due to
View Solution



