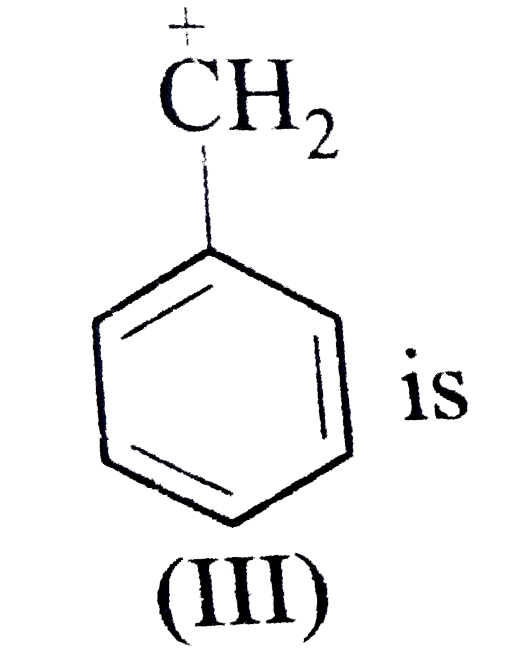
What si the correct order of decreasing stability of the following carbocations.
CH3−⊕CH−CH3I. CH3−⊕CH−OCH3II.
CH3−⊕CH−CH2−OCH3III.
Video Solution
Text Solution
(i) HCl (aq) - Acid (Arrhenius conecpt and Bronsted - Lowry concept)
(ii) NH3(g)-Base (Bronsted concept and Lewis concept)
(iii) Na2CO3(aq)- Base (Bronsted concept)
(iv) CH3COOH(aq)-Acid (Arrhenius concept and Bronsted concept)
(v) CO2(g)-Acid (Bronsted concept and Lewis concept)
(vi) BF3-Acid (Lewis concept)
(vii) Ag+- Acid (Lewis concept)
(viii) CN−- Base (Lewis concept)
(ix) H2O- Both acid and base, i.e., amphoteric (Bronsted concept)
(x) H2SO4- Both acid and base, i.e., amphoteric (Bronsted concept)
(xi) CHO−3- Both acid and base, i.e., amphoteric (Bronsted concept)
(xii) SiF4 - Acid (Lewis concept), as silicon can expand its octet .
|
Topper's Solved these Questions
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Conceptual Questions ( III. Ionic product of water and pH)
Explore 5 Videos
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Conceptual Questions (IV. Salt hydrolysis)
Explore 1 Video
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Conceptual Questions (I. Storng and weak electrolytes, ionic equilibrium and Ostwalds dilution law)
Explore 1 Video
- ENVIRONMENTAL CHEMISTRYBook:PRADEEPChapter:ENVIRONMENTAL CHEMISTRYExercise:COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II
Explore 6 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
Similar Questions
Knowledge Check
What si the correct order of decreasing stability of the following carbocations.
CH3−⊕CH−CH3I. CH3−⊕CH−OCH3II.
CH3−⊕CH−CH2−OCH3III.AII>I>IIIBII>III>ICIII>I>IIDI>II>IIIWhat is the correct order of decreasing stability of the following cations?
CH3−+CH−CH3I CH3−+CH−OCH3II
CH3−+CH−CH2−OCH3IIIAIIgtIgtIIIBIIgtIIIgtICIIIgtIgtIIDIgtIIgtIIIThe decreasing order of the stability of the ions :
CH3=+CH−CH3(I) CH3−+CH−OCH3(II) CH3−+CH−COCH−3(III)AI>II>IIIBIII>II>ICII>III>IDII>I>III
What is the correct order of decreasing stability of the following cations
H3C−⊕CH(I)−CH3 , H3C−⊕CH(II)−OMe
H3C−⊕CH(III)−CH2−OMe
View SolutionWhat is the correct order of decreasing stability of the following cations
I. CH3−⊕CH−CH3 II. CH3−⊕CH−OCH3
III. CH3−⊕CH−CH2−OCH3
View SolutionThe decreasing order of the stability of the ions
CH3−+CH−CH3(I), CH3−+CH−OCH3(II), , CH3−+CH−COCH3(III),
View SolutionWhat is the correct order of decreasing stability of the following cations ?
⊕CH3−CH−CH3
II ⊕CH3−CH−OCH3
III ⊕CH3−CH−CH2−OCH3
View SolutionThe correct decreasing order of stability of I,II and III carbocations is:
(I) CH3−+CH−CH3 (II) CH3−+CH−COCH3
(III) CH3−+CH−OCH3
View Solution