On the basis of Le Chatelier principle explain how temperature and pressure can be adjusted to increase the yield of ammonia int he following reaction.
N2(g)+3H2(g)⇔2NH3(g),ΔH=−92.38KJMol−1
What will be the effect of addition of argon to the avove mixture at cosntant volume ?
Step by step video solution for On the basis of Le Chatelier principle explain how temperature and pressure can be adjusted to increase the yield of ammonia int he following reaction. N_2(g)+3H_2(g)hArr2NH_3(g), DeltaH=-92.38KJ Mol^(-1) What will be the effect of addition of argon to the avove mixture at cosntant volume ? by Chemistry experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Additional Questions (Very short answer questions) (I. Strong and weak electrolytes, ionic equilibrium and Ostwalds dilution law)
Explore 1 Video
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Additional Questions (Very short answer questions)
Explore 1 Video
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:NCERT Exemplar Problems with answers, Hints and Solutions (Assertion and Reason Type Questions )
Explore 7 Videos
- ENVIRONMENTAL CHEMISTRYBook:PRADEEPChapter:ENVIRONMENTAL CHEMISTRYExercise:COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II
Explore 6 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
Similar Questions
Knowledge Check
For the reaction
N2(g)+3H2(g)⇔2NH3(g),ΔH=?AΔE+2RTBΔE−2RTCΔE+RTDΔE−RTWhich of the following factors favour the reaction:
N2(g)+3H2(g)⇔2NH3(g)ΔH=-93.6 kJAincrease in pressureBincrease in temperatureCdecrease in temperatureDBoth A and CThe % yield of ammonia as a function of time in the reaction N2(g)+3H2(g)⇔2NH3(g),(g)ΔH<0 at (P,T1) is given below
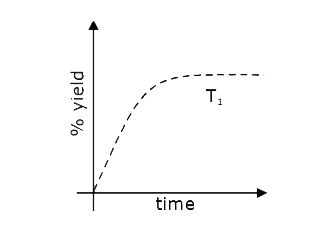 A
A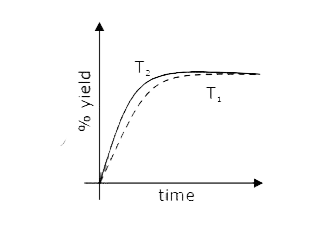 B
B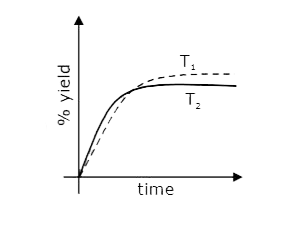 C
C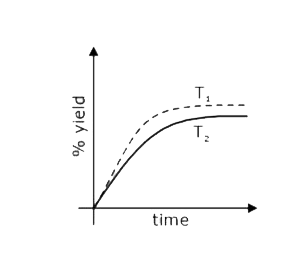 D
D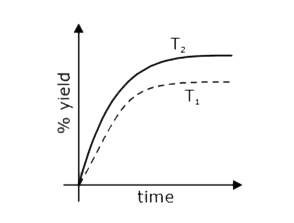
Which of the following is correct for the reaction?
N2(g)+3H2(g)⇔2NH3(g)
View SolutionIn reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJ/mole, the yield of ammonia does not increase when
View SolutionIn reaction N2(g)+3H2(g)⇔2NH3(g),ΔH=−93.6kJ/mole, the yield of ammonia does not increase when
View SolutionWhich of the following is correct for the reaction?
N2(g)+3H2(g)⇔2NH3(g)
View SolutionFor the reaction N2(g)+3H2(g)→2NH3(g),ΔH=−93.6KJmol−1 the formation of NH3 is expected to increase at :
View Solution
