Universal indicator shows green colour when pH of the solution is nearly....... .
4
11
12
7
The correct Answer is:D
To solve the question regarding the pH at which a universal indicator shows a green color, we can follow these steps:
1. Understanding pH Scale: The pH scale ranges from 0 to 14, where:
- pH < 7 indicates acidity,
- pH = 7 indicates neutrality,
- pH > 7 indicates alkalinity.
2. Identifying the Universal Indicator: A universal indicator is a pH indicator that shows different colors at different pH levels. It is used to determine the acidity or alkalinity of a solution.
3. Color Correspondence: The universal indicator shows specific colors at certain pH levels:
- Red for acidic solutions (pH < 7),
- Green for neutral solutions (pH = 7),
- Blue to purple for alkaline solutions (pH > 7).
4. Conclusion: Since the question asks when the universal indicator shows green, we can conclude that this occurs at a pH of approximately 7.
5. Final Answer: Therefore, the universal indicator shows green color when the pH of the solution is nearly 7.
- pH < 7 indicates acidity,
- pH = 7 indicates neutrality,
- pH > 7 indicates alkalinity.
- Red for acidic solutions (pH < 7),
- Green for neutral solutions (pH = 7),
- Blue to purple for alkaline solutions (pH > 7).
Topper's Solved these Questions
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Competition Focus (VII. Numerical Value Type Questions) (In Decimal Notation)
Explore 1 Video
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Competition Focus (VIII. Assertion-Reason Type Questions)
Explore 16 Videos
- EQUILIBRIUMBook:PRADEEPChapter:EQUILIBRIUMExercise:Competition Focus (V. Matrix-Match Type Questions)
Explore 2 Videos
- ENVIRONMENTAL CHEMISTRYBook:PRADEEPChapter:ENVIRONMENTAL CHEMISTRYExercise:COMPETITION FOCUS (JEE(Main and Advanced)/Medical Entrance (VI.ASSERTION-REASON) Type II
Explore 6 Videos
- EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESBook:PRADEEPChapter:EQUILIBRIUM IN PHYSICAL AND CHEMICAL PROCESSESExercise:Competition Focus (Jee(Main and advanced)/Medical Entrance) VIII. ASSERTION - REASON TYPE QUESTIONS (TYPE - II)
Explore 10 Videos
Similar Questions
A salt is dissolved in water and the pH of this salt solution is measured with a universal indicator paper. If the pH of solution is less than 7, the salt is most likely to :
View SolutionWhat colour will universal indicator show if you add it to the following substnaces?
(a). Potassium hydroxide pH=12
(b). Soda water, pH=5
(c). Sulphuric acid, pH=2.
View Solution
Knowledge Check
The colour of methyl orange indicator in a solution is yellow. The pH of this solution is likely to be:
A7Bless than 7C0Dmore than 7A solution showed pH 9. This solution is:
AStrongly acidicBStrongly alkalineCWeakly acidicDWeakly alkaline.The indicator that shows colour changes in the entire pH range is
AphenolphthaleinBmethyl orangeCuniversal indicatorDthymol blue
On adding a few drops of universal indicator to three unknown colourless solutions (P), (Q) and (R ) taken separately in three test tubes shown in the following diagrams, a student observed the changes in colour as green in (P ), red in (Q) and violet in (R ). What will be the decreasing order of pH ?
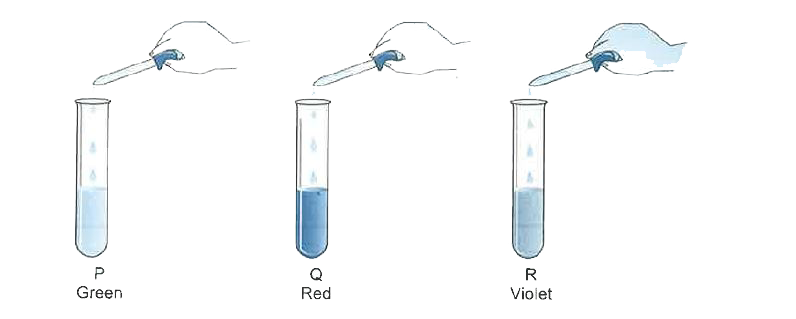
View Solution(a). What would you expect the pH of pure water to be?
(b). What colour would the universal indicator show in an aqueous solution of sugar? Why?
(c). A sample of rain water turned universal indicator paper yellow. What would you expect its pH to be? Is it a strong or a weak acid?
View SolutionThe indicator which produces a pink colour in an alkaline solution is:
View SolutionOn adding a few drops of universal indicator solution to three unknown colourless solutions P,Q, and R taken separately in three test-tubes shown in the diagrams, a student observed the changes in colour as green in solution P red in solution Q and violet in solution R.
The decreasing order of the pH of the three solutions is:
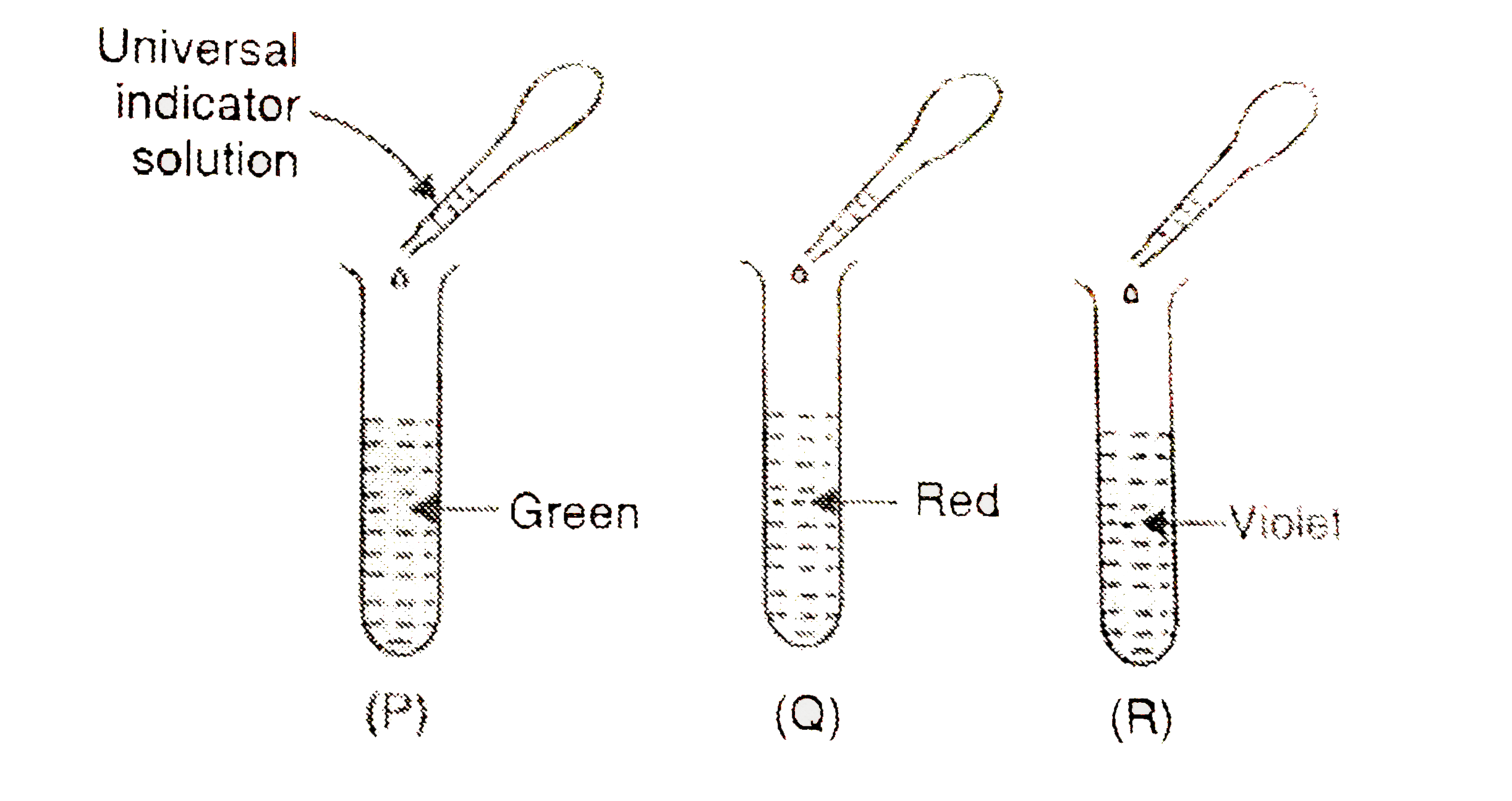
View SolutionIn an experiment to measure the pH values by using a universal a universal indicator a student found that the pH values of four given solution A,B,C and D are 10,6,2 and 14 respectively. The solution which will require the maximum volume of sodium hydroxide solutioin for complete neutralisation is solution:
View Solution
