The compound that does not produce nitrogen gas by the thermal decomposition is
A
Ba(N3)2
B
(NH4)2Cr2O7
C
NH4NO2
D
(NH4)2SO4
वीडियो समाधान
लिखित उत्तर
The correct Answer is:D
Only (NH4)2SO4 on heating does not give N2 but gives NH3
(NH4)2SO4⇔2NH3+H2SO4
|
टॉपर्स ने हल किए ये सवाल
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:Competition focus jee(main and advanced)/ medical entrance special) (II. MULTIPLE CHOICE QUESTIONS (with One or More than One Correct Answers))
Explore 8 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:Competition focus jee(main and advanced)/ medical entrance special) (III. multiple choice questions (based on the given Passage/Comprehension))
Explore 2 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)Book:PRADEEPChapter:P-BLOCK ELEMENTS (NITROGEN FAMILY)Exercise:ANALYTICAL QUESTIONS AND PROBLEMS WITH ANSWERS/SOLUTIONS (PROBLEMS)
Explore 4 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBook:PRADEEPChapter:ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESExercise:Competition Focus (Jee (Main and Advanced)/Medical Entrance) VIII. Assertion-Reason Type Questions
Explore 25 Videos
- REDOX REACTIONS Book:PRADEEPChapter:REDOX REACTIONS Exercise:Assertion reason type question
Explore 16 Videos
समान प्रश्न
ज्ञान जांच
The compound that does not produce nitrogen gas by the thermal decomposition is
ABa(N3)2B(NH4)2Cr2O7CNH4NO2D(NH4)2SO4The compound that does not produce nitrogen gas upon thermal decomposition is :
A(NH2)2SO4BBa(N3)2C(NH4)2Cr2O7DNH4NO2The reaction that doses not produce nitrogen is :
Aheating (NH4)2Cr2O7BNH3 with excess of Cl2Cheating of NaN3Dheating of NH4NO3
A neon gas does not produce :
View SolutionNitrogen is obtained by the thermal decomposition of :
View SolutionNitrogen gas is liberated by thermal decomposition of:
View SolutionWhich of the following compound does not produce green coloured product on thermal decomposition?
View SolutionWhich of the following compound does not produce green coloured product on thermal decomposition?
View Solution

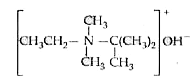 gives
gives