A hypothetical elecrochemical cell is shown below:
Aɵ∣∣A+(xM)∣∣∣∣B+(yM)∣∣∣B⊕
The emf measured is +0.20V. The cell reaction is
A
The cell reaction cannot be predicted
B
A+B_→A++B
C
A++B→A+B+
D
A++e−→A,B++e−→B
Video Solution
Text Solution
The correct Answer is:B
ΘA/A+(xM) represents -ve pole (anode) on which oxidation occurs. B+(yM)/⊕B represents +ve pole (cathode) on which reduction occurs. Hence, cell reaction is A+B+→A++B
|
Topper's Solved these Questions
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:TEST YOUR GRIP (FILL IN THE BLANKS)
Explore 29 Videos
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:CONCEPTUAL QUESTIONS
Explore 46 Videos
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:Advanced Problem For Competitions
Explore 17 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:IMPORTANT QUESTIONS
Explore 30 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS Book:PRADEEPChapter:GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS Exercise:Curiosity Questions
Explore 2 Videos
Similar Questions
Knowledge Check
A hypothetical electrochemical cell is shown below.
A∣∣A+(xM)∣∣∣∣B+(yM)∣∣B+
The e.m.g. measured is 0.20V the cell reaction isAThe cell reaction cannot be predictedBA+B+→A++BCA++B→A+B+DA++e−→A:B++e−→B.A hypothetical elecrochemical cell is shown below:
Aɵ∣∣A+(xM)∣∣∣∣B+(yM)∣∣∣B⊕
The emf measured is +0.20V. The cell reaction isAThe cell reaction cannot be predictedBA+B_→A++BCA++B→A+B+DA++e−→A,B++e−→BA hyptherical electrochemical cell is given as AӨ∣∣A+(xM)∣∣∣∣B+(yM)∣∣BӨ The emf masured is +0.20V. The cell reaction is .
AThe cell raction cannot be prdictedB1A+B+→A++BCA++B→A+B+DA++e−→A,V+e−→B
A hypothetical electrochemical cell shown below
⊕A|(xM)|∣∣B+(yM)∣∣ ⊕B
The e.m.f. measured is +0.20 V . The cell reaction is :
View SolutionEo for the reaction Fe+Zn2+→Zn+Fe2+ is –0.35 V. The given cell reaction is :
View SolutionFor a hypothetical reaction
A+B→C if ΔG∘>0
View SolutionFind the power supplied by 20V cell in the figure shown.
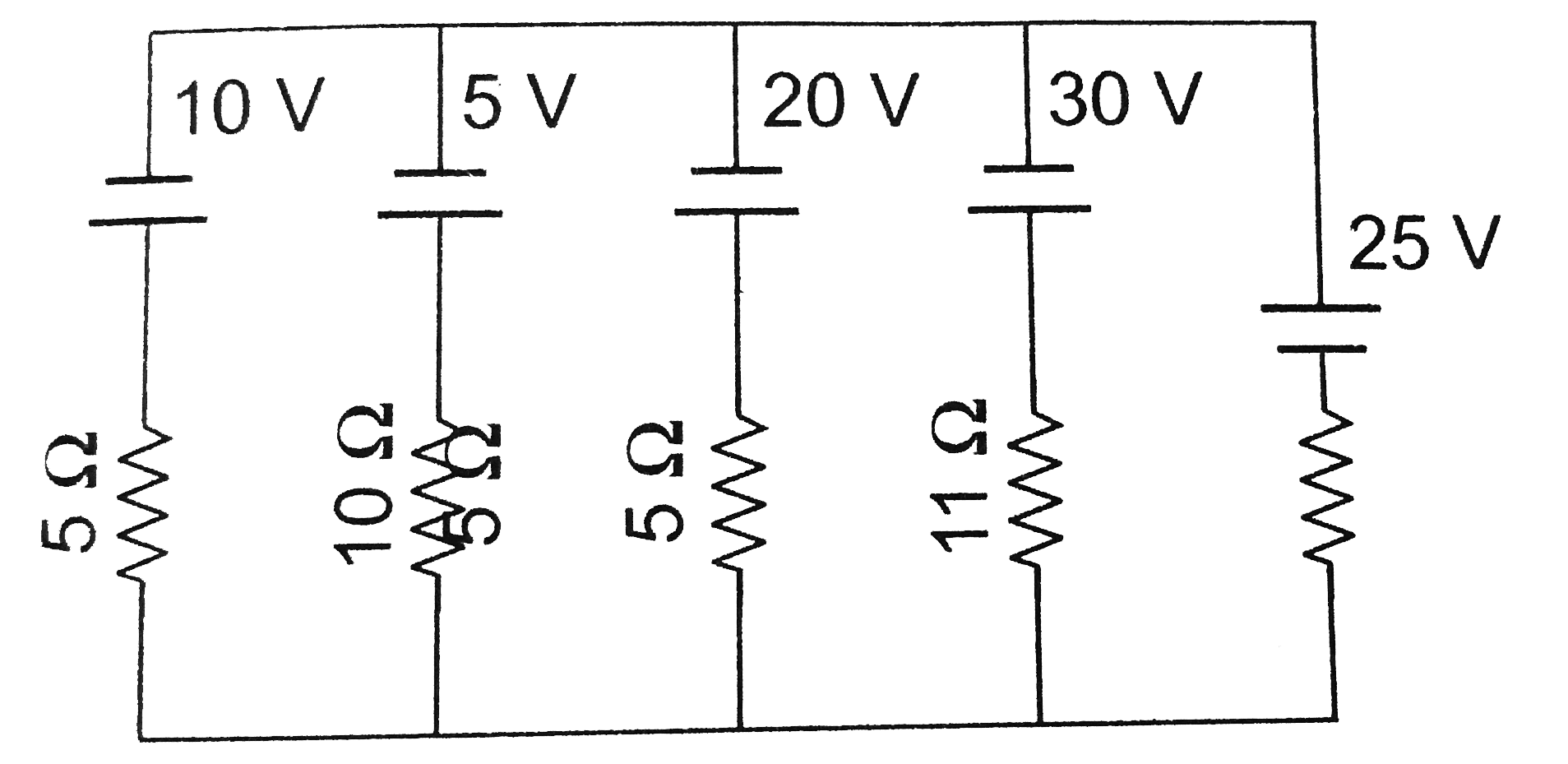
View SolutionA secondary cell have source emf of 3 V. When this cell is connected to a load of 0.25 then load emf calculated is 2.10 V. The internal resistance of cell and power consumed are
View Solution
