CALOMEL ELECTRODE
Hg2Cl2(s)+2e−→2Hg(l)+2Cl−(aq)
टॉपर्स ने हल किए ये सवाल
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - CONCEPTUAL QUESTIONS
46 Videos
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - NCERT QUESTIONS AND EXERCISES WITH ANSWER
15 Videos
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - TEST YOUR GRIP (MUTIPLE CHOICE QUESTION)
20 Videos
- D- AND F-BLOCK ELEMENTSBOOK - PRADEEPCHAPTER - D- AND F-BLOCK ELEMENTSEXERCISE - IMPORTANT QUESTIONS
30 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS BOOK - PRADEEPCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS EXERCISE - Curiosity Questions
2 Videos
समान प्रश्न
A saturated calomel electrode is coupled through a salt bridge with a quinhydrone electrode dipping in 0.1MNH4Cl. The observed EMF at 25∘C is 0.152V. Find the dissociated constant of NH4OH. The oxidation potential of saturated calomel electrode=−0.699V at 25∘C.
View SolutionStandard Electrode Potential||Half Cells||their reactions and representation||Complete Galvanic Cell with reactions and representations||Reference Electrodes||Standard Hydrogen Electrode||Secondary Electrodes Calomel Electrode and Silver Electrode
View Solution
ज्ञान जांच
Match the List-I with List-II
List-I (Electrode)List -II (Type)1Calomel(A)Reference2Glass(B)Redox3Hydrogen(C)Membrane4Quinhydrone(D)GasA1−A,2−C,3−D,4−BB1−B,2−A,3−D,4−CC1−C,2−B,30A,4−DD1−D,2−A,3−C,4−BMatch the list - I with List - II
List (Electrode)List - II(Type)1.CalomelP.Reference2.GlassQ.Redox3HydrogenR.Membrane4.QuinhydroneS.GasA1-P,2-R,3-S,4-QB1-Q,2-P,3-S,4-RC1-R,2-Q,3-P,4-SD1-S,2-P,3-R,4-QCalomel (Hg2Cl2) on reaction with ammonium hydroxide gives
AHgNH2ClBNH2−Hg−Hg−ClCHg2ODHgO
The cell, Pt|H2 (1 atm)|∣∣H+(pH=x)∣∣
normal calomel electrode, has an emf of 0.67 volt as 25∘C. Calculate the pH of the solution. The oxidation potential of calomel electrode on the hydrogen scale is -0.28 volt.
View SolutionA certain electrode has a standard reduction potential of 0.140V when measure against a saturaed calomel electrode (E∘RP=+0.244V). Calculate its standard reduction potential against a standard H-electrods.
View SolutionThe quinhydrone electrode (Q,H⊕∣H2Q) is used in conjunction with a saturated calomel electrode, as represented below:
EMFcell=0.264V at 30∘C. Calculate the pH of unknown solution at this temperature.
Given :Ecalomel=0.24V and Ec−.2H⊕,Q∣H2Q=0.7V
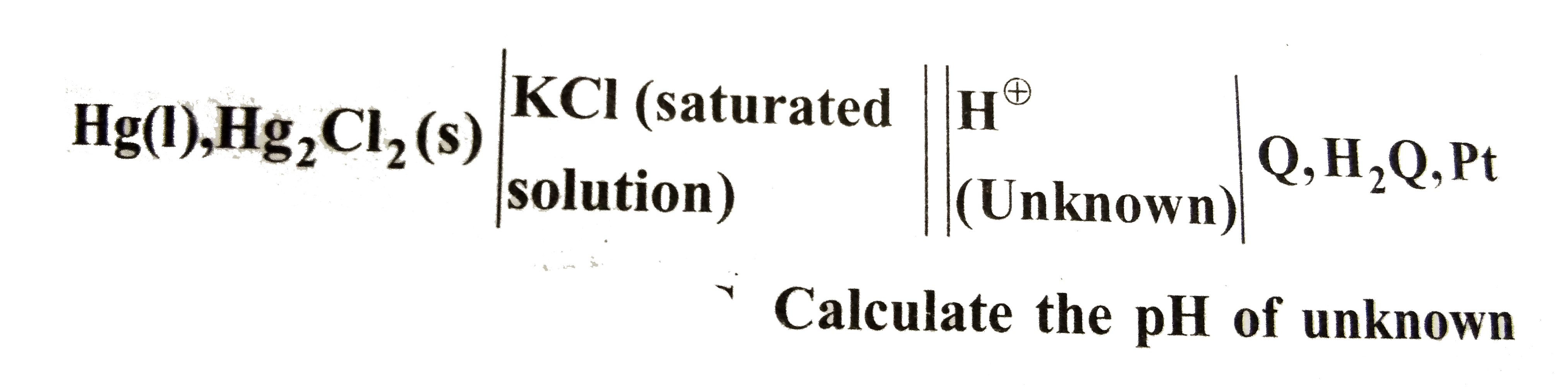
View SolutionCalomel (Hg2Cl2) on reaction with ammonium hydroxide gives
View SolutionCalomel (Hg2Cl2) on reaction with ammonium hydroxide gives
View Solution

