AgNO3(aq) was added to an aqueous KCl soluton gradually and the coductivity of the solution was measured. The plot of conductanec (∧) versus the volume of AgNO3 is
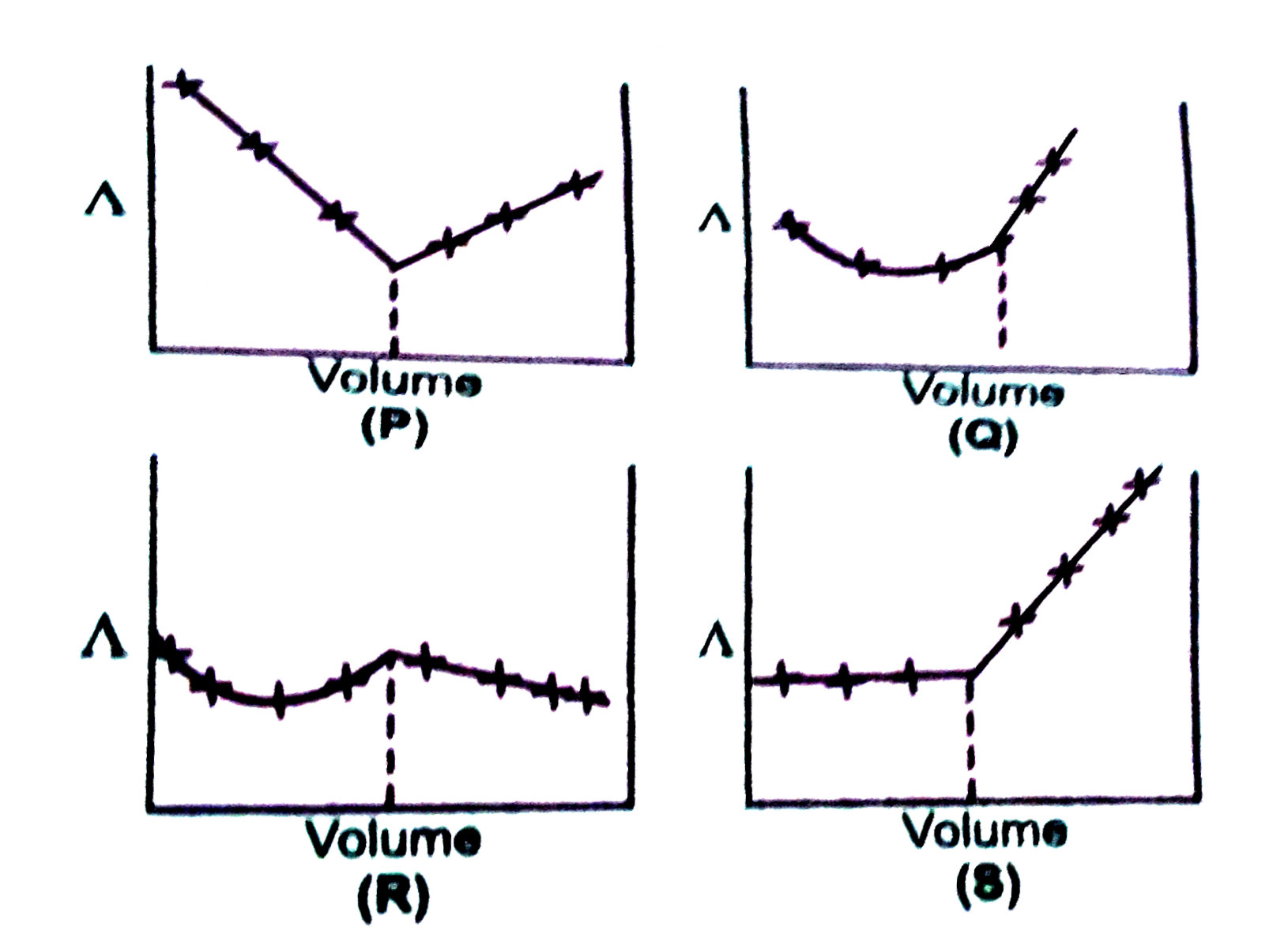
(P)
(Q)
(R)
(S)
The correct Answer is:D
[K+(aq)+Cl−(aq)]+Ag+(aq)+NO−3(aq)→K+(aq)+NO−3(aq+AgCl(s)
Initially the conductance is due to K+ and Cl− ions. On dding AgNO3, precipitate of AgCl is formed. Thus, some Cl− ions are replaced by NO−3 ions. As both have nearly the same conductance, the conductance remains almost constant. after the end point, addition of AgNO3
Will give Ag+andNO−3 ions in the solution. hence, conductance will increase rapidly.
Topper's Solved these Questions
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-II)
7 Videos
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - VALUE BASED QUESTIONS WITH ANWER (Multiple Choice Questions-III Based on the given Passage/Comprehension)
13 Videos
- ELECTROCHEMISTRYBOOK - PRADEEPCHAPTER - ELECTROCHEMISTRYEXERCISE - HIGHER ORDER THINKING SKILLS (HOTS PROBLEMS)
13 Videos
- D- AND F-BLOCK ELEMENTSBOOK - PRADEEPCHAPTER - D- AND F-BLOCK ELEMENTSEXERCISE - IMPORTANT QUESTIONS
30 Videos
- GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS BOOK - PRADEEPCHAPTER - GENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS EXERCISE - Curiosity Questions
2 Videos
Similar Questions
Which of the following statements are not correct?
View SolutionThere are two principal types of electrochemical cells. A galvanic cell is an electrochemical cell that produces electricity as a result of spontaneous reaction occurring inside it. An electrolytic cell is an electrochemical cell in which a non-spontaneous reaction is driven by an external source of current. any redox reaction may be expressed in terms of two half reactions which are conceptual reactions showing the lowss and gain of electrons. each half reaction has a difinite value of standard electrode potential. the overall reaction is represented by a universally accepted method. knowing the standard electrode potential of the half reactions, the standard EMF of the cell can be calculted. the standard EMF further helps in the calculation of free energy change, equilibrium constant of the cell reaction as well as parameters like solublity products of a sparingly soluble salt. a cell can also be set up in which the two electrodes may be of the same (type, e.g., both may be hydrogen electrodes but the concentration of H+ ions in the two solutions may be different. Such cells are called concentration cells.
Q. The reaction
12H2(g)+AgCl(s)→H+(aq)+Cl−(aq)+Ag(s)
occurs in the galvanic cell
View SolutionCalculate the stability constant of the complex [Zn(NH3)4]2+ formed in the reaction
Zn2++4NH4⇔[Zn(NH3)4]2+.
Given that E∘Zn2+/Zn=−0.76VandE∘(Zn(NH3)4]2+/Zn,4NH3)∘=−1.03V
View SolutionSilver atom has completely filled d orbitals (4d10) in its ground state. How can you say it is a transition element?
View SolutionUsually a broken iron article is taken to the welding shop for repair. But it is difficult to take broken girders or the craked railway track to the industry for repair. Can the welding equipment be brought to the site for repair ? Name the process, illustrate the principle of its working and name some other practicle applications of this process.
View SolutionAgNO2(aq) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot conductivity of the solution was measured. The plot of conductance (∧) versus the volume of AgNO3 is
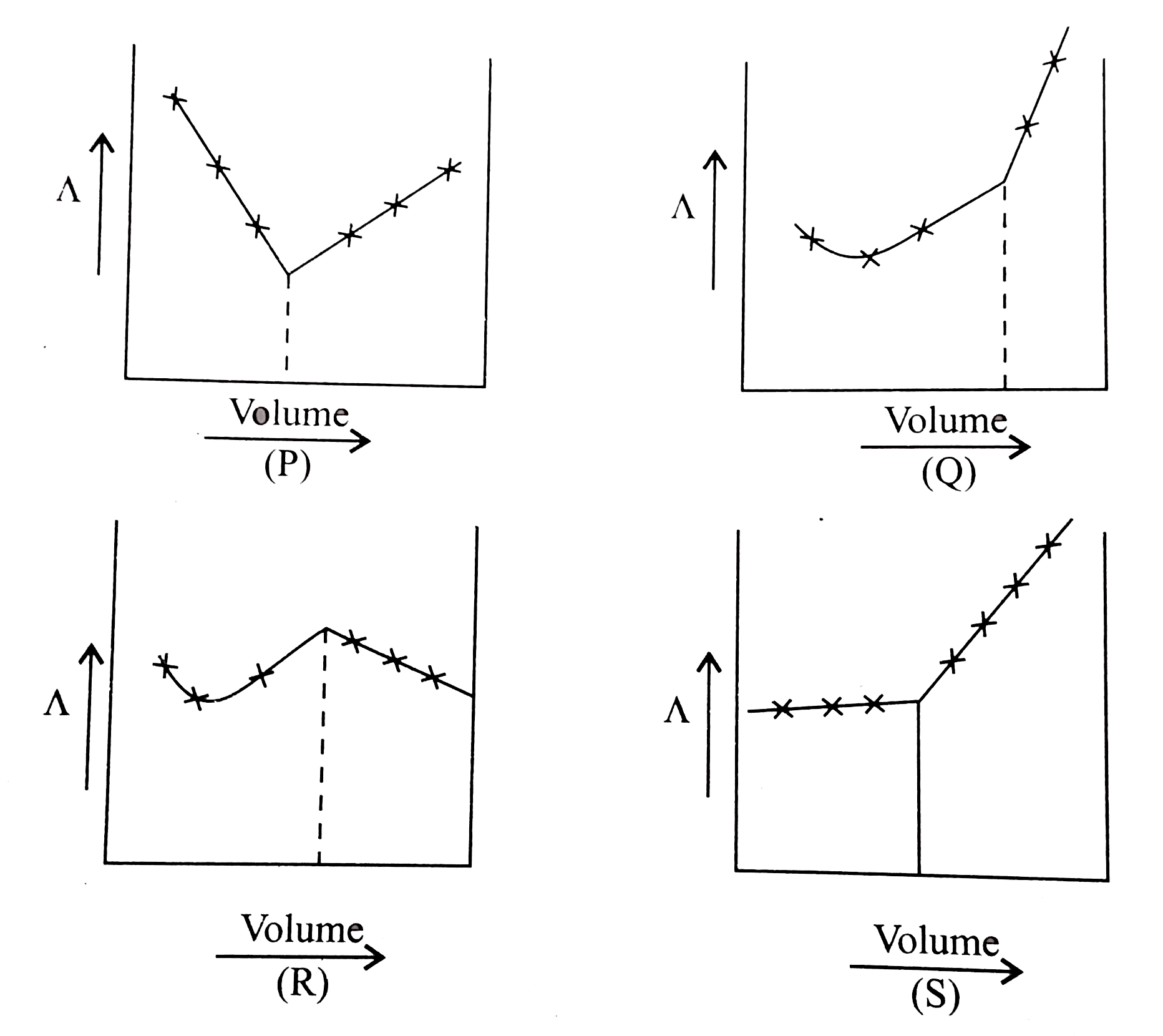
View SolutionAgNO3(aq.) was added to an aqeous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance (Λ) versus the volume of AgNO3 is :
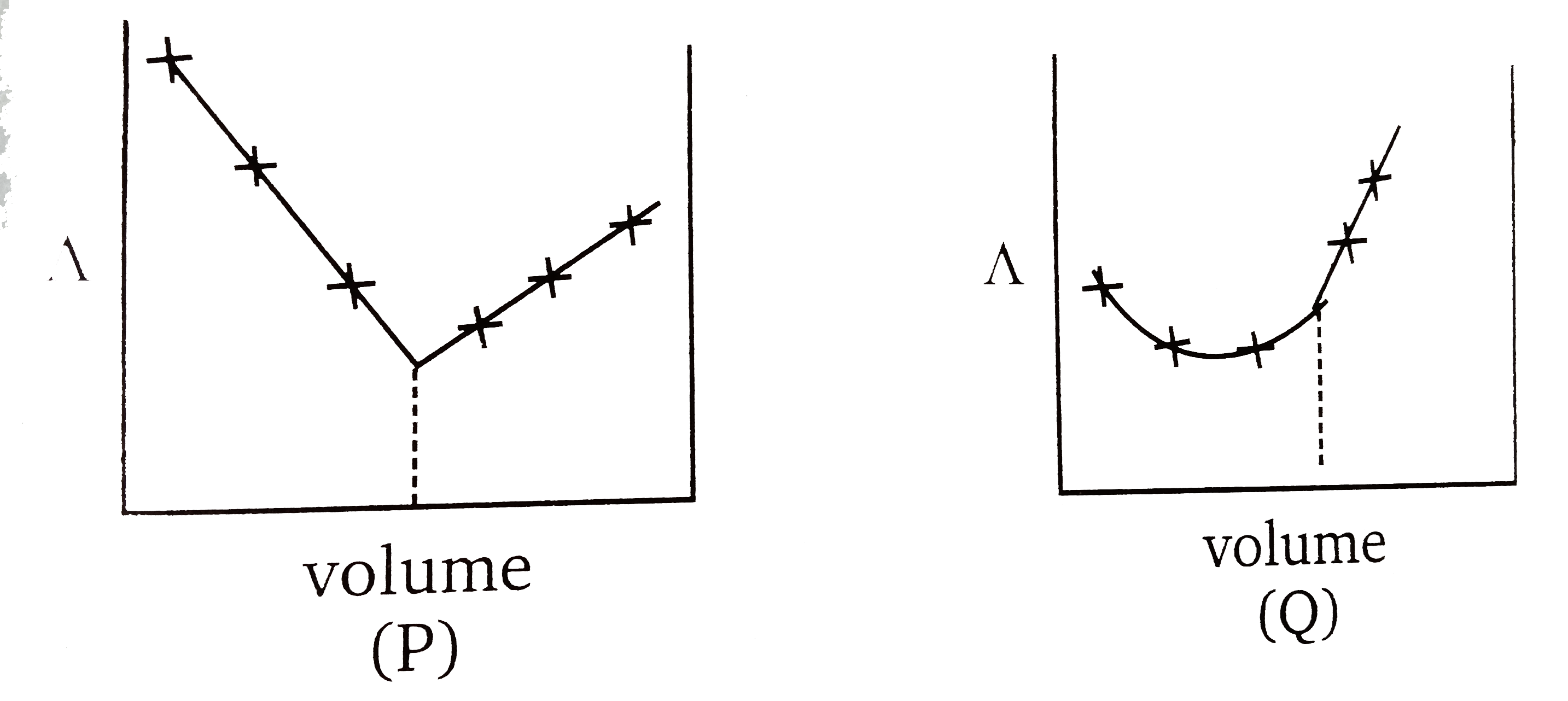
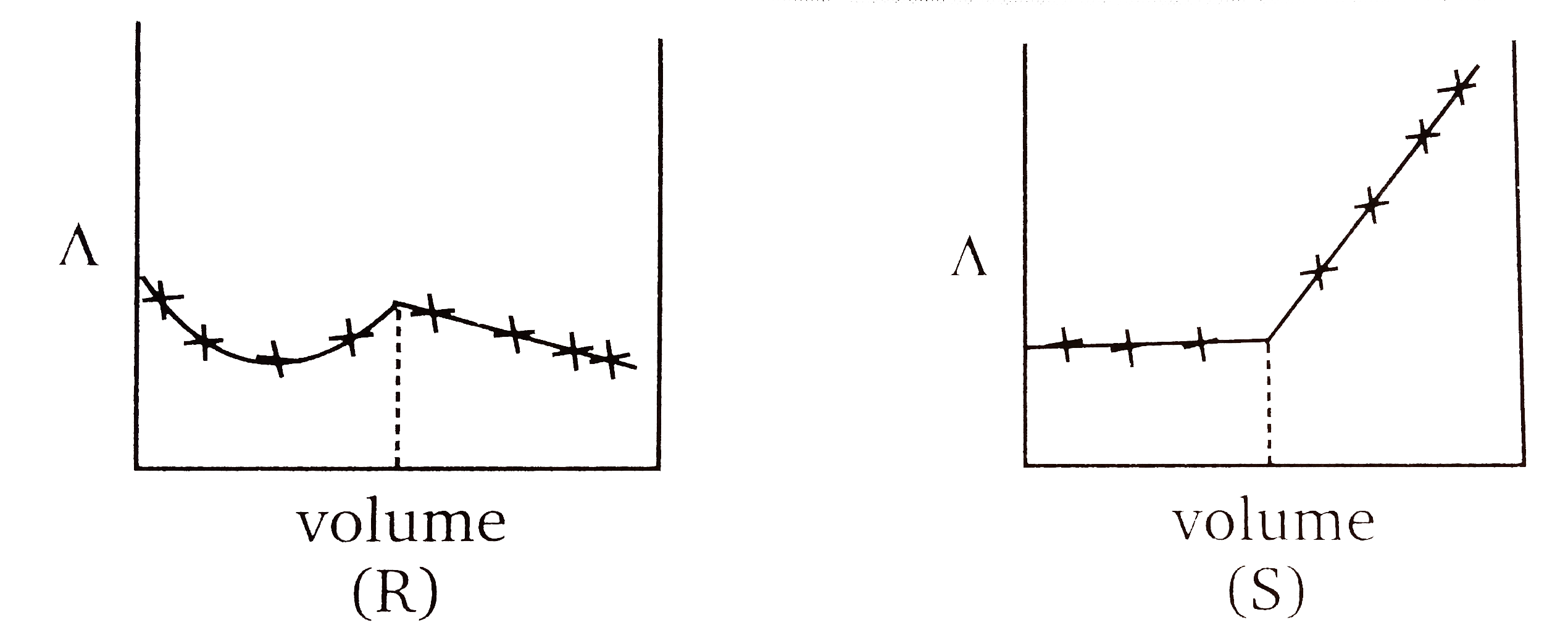
View SolutionTo prepare a solution of concentrated of 0.03g/mlof AgNO3,what amount of AgNO3 should be added in 60ml of solution
View SolutionTo prepare a solution of concentration of 0.03 g/ml. of AgNO3. What amount of AgNO3 should be added in 60mL of solution?
View SolutionAgNO3 (aq) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance (∧) versus the value of AgNO3 is
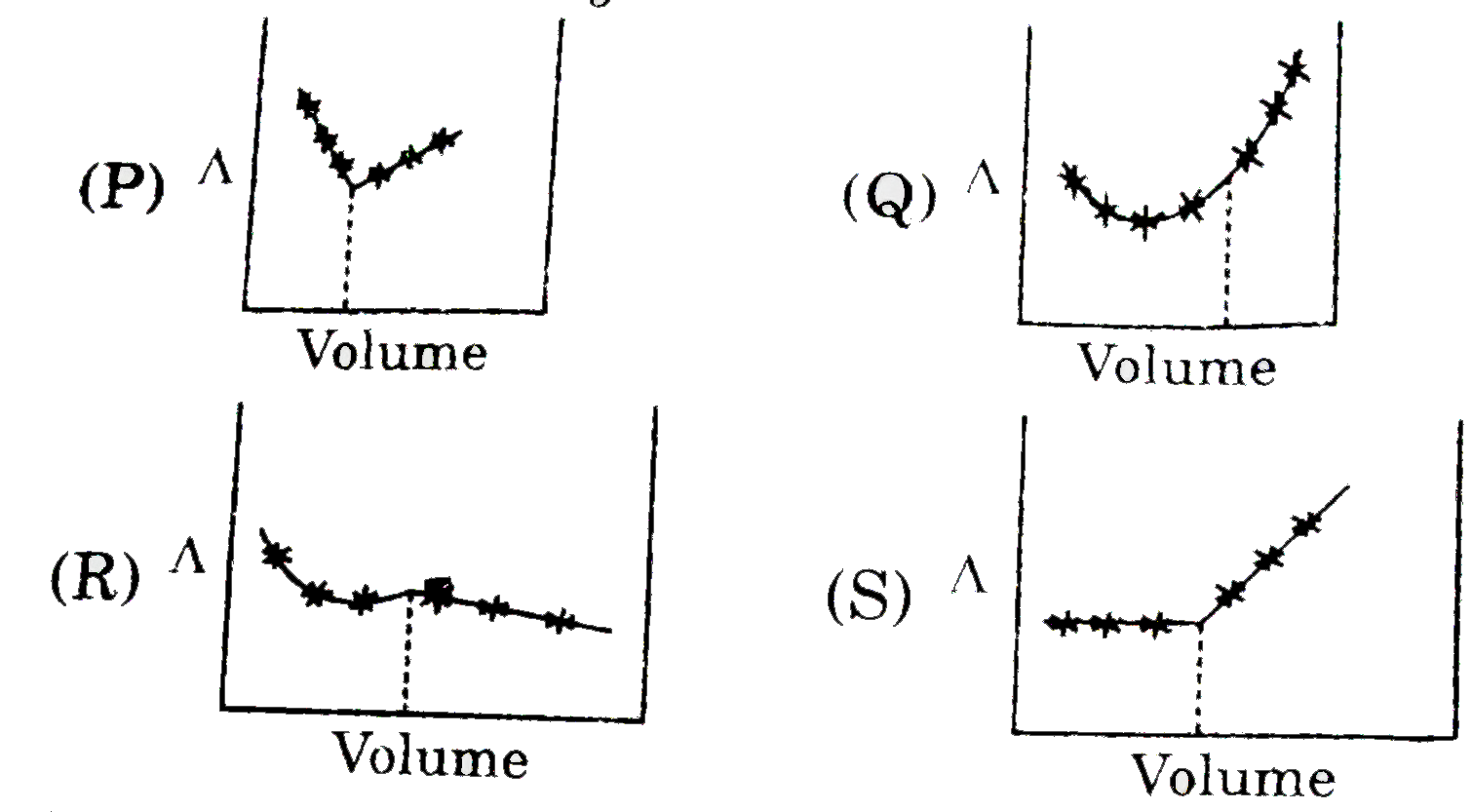
View SolutionAgNO_(3)(aq) was added to an aqueous KCl soltuion gradually and conductivity of the solution was measured. The plot of conductance (A) versus the value of AgN O_(3) is
View SolutionAgNO3(aq) was added to an aqueous KCl solution gradully and the conductivity of the solution was measured. The plot of conductance (Λ) versus the volume of AgNO3 is

View SolutionType of collcidal solutions result when AgNO3
Solution is added to Kl solution and when Kl solution
is added so AgNO3 soultion are respectively
View SolutionAgNO_(3) (aqueous) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance (A) versus the volume of
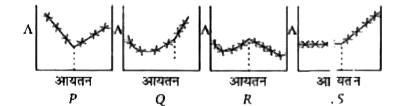
View Solutionजलीय KCl विलयन में क्रमश: जलीय AgNO3(aq) डालते हुए विलयन की चालकता मापी गयी | AgNO3 के आयतन तथा चालकता (A) के मध्य ग्राफ है
View SolutionAgNO3(aq) was added to an aqueous KCl solution gradually and the conductivity of the solution was measured. The plot of conductance A versus the volume of AgNO3 is

View Solution

