Describe giving reason which one of the following pairs has the property indicated?
(a). Fe or Cu has higher melting point.
(b). Co2+ or Ni2+ has lower magnetic moment.
Video Solution
Text Solution
(i) Fe has higher melting point than Cu. This is because Fe has four unpaired electrons in 3d -subshell while Cu has only one electron in the 4s-subshell. Hence, metallic bonds in Fe are much stronger than those in CU.
(ii) .27Co=[Ar]3d74s2,Co2+=[Ar]3d7(3 unpaired electron)
.28(Ni)=[Ar]3d84s2,Ni2+=[Ar]3d8(2 unpaired electrons)
Hence, Ni has lower magnetic moment than Co.
|
Topper's Solved these Questions
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:NCERT (QUESTIONS AND EXERCISES WITH ANSERS)
Explore 10 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:NCERT INTEXT UNSOLVED QUESTIONS
Explore 10 Videos
- D- AND F-BLOCK ELEMENTSBook:PRADEEPChapter:D- AND F-BLOCK ELEMENTSExercise:TEST YOUR GRIP( FILL IN THE BLANKS)
Explore 33 Videos
- CORDINATION COMPOUNDSBook:PRADEEPChapter:CORDINATION COMPOUNDSExercise:Important Questions
Explore 28 Videos
- ELECTROCHEMISTRYBook:PRADEEPChapter:ELECTROCHEMISTRYExercise:IMPORTANT QUESTIONS FOR BOARD EXAMINATION
Explore 25 Videos
Similar Questions
Knowledge Check
Which of the following pairs has bleaching property
AO3 and NO2BO3 and H2SCSO2 andCl2DCl2 and NO2Which one of the following has the lowest melting point ?
ANBPCAsDSbWhich of the following pairs has zero dipole moment ?
ACH2Cl2andNF3BSiF4andBF3CPCl3andClFDBF3andNF3
Which of the following pairs has higher resonance energy :
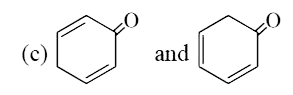
View SolutionWhich of the following pairs has higher resonance energy :
View SolutionWhich one of the following has the least magnetic moment?
View SolutionWhich one of the following has the least magnetic moment ?
View SolutionWhich one of the following has the highest melting point ?
View Solution
