(a) List two limitations of Newlands' Law of Octaves.
(b) Write the electronic configuration of two elements A and B whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element A reacts with element B. State whether this compound is acidic, basic or neutral. Give reason to justify your answer.
(a) Limitations of Newlands' Law of Octaves :
(i) All the elements discovered at that time could not be classified into Octaves. The law was applicable only upto calcium, as after calcium every eighth element did not posses properties similar to that of the first.
(ii) Newland did not take into account the elements yet to be discovered. The newly discovered elements could not fit into his table.
(b) Electronic configuration of elements 'A' and 'B' :
A(20 ) ⇒ 2, 8, 8, 2
B (17) ⇒ 2, 8, 7
Molecular formula of compound formed when element A reacts with elements B: AB2
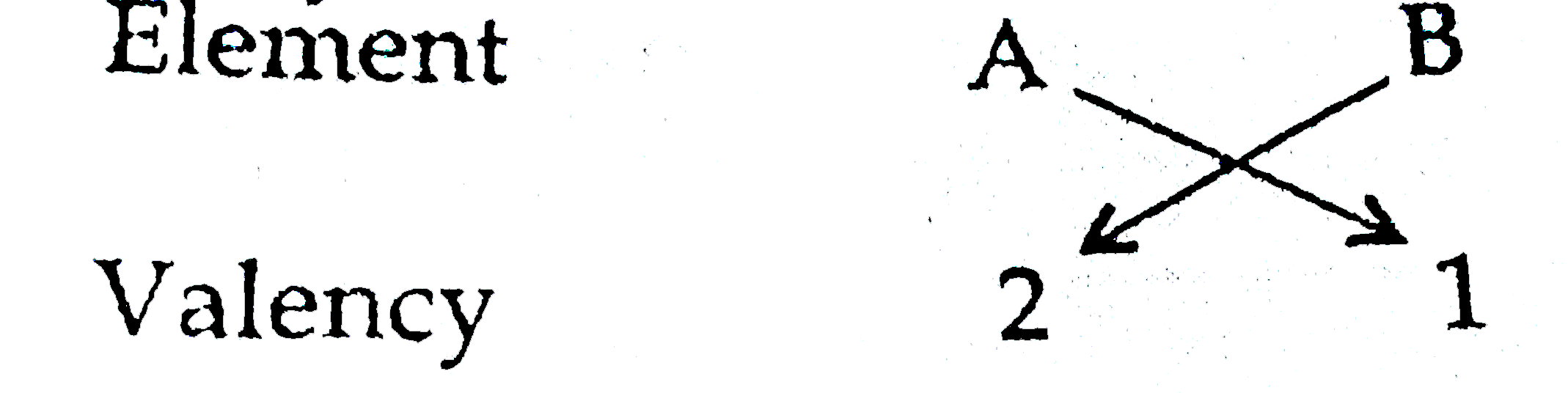
It is a neutral salt. It is a salt of a strong acid and a strong base.
Topper's Solved these Questions
- X BOARDSBOOK - X BOARDSCHAPTER - X BOARDSEXERCISE - SET III (SECTION -B)
1 Video
- X BOARDSBOOK - X BOARDSCHAPTER - X BOARDSEXERCISE - SET III (SECTION -C)
1 Video
- X BOARDSBOOK - X BOARDSCHAPTER - X BOARDSEXERCISE - SET II (SECTION -C)
1 Video
- QUESTION PAPER 2023BOOK - X BOARDSCHAPTER - QUESTION PAPER 2023EXERCISE - Question
24 Videos
Similar Questions
"Conversion of ethanol of ethanoic acid is an oxidation reaction . " Justify this statement giving the relevant equation for the chemical reaction involved .
View SolutionA metal X ,which is used in thermit process , when heated with oxygen gives an oxide Y which is amphoteric in nature . Identify X and Y. Write balanced chemical equation of the reaction of oxide Y with hydrochloric acid and sodium hydroxide .
View SolutionSalt 'P', commonly used in bakery products, on heating gets converted into another salt 'Q' which itself is used for the removal of hardness of water and a gas 'R' is evolved. The gas 'R' when passed through freshly prepared lime water turns milky. Identify 'P', 'Q' and 'R', giving chemical equation for the justification of your answer.
View SolutionThe correct representation of covalent bonding in an oxygen molecule is:
View SolutionElements with atomic numbers 17 and 20 form compounds with hydrogen. Write the formula of these two compounds and compare their chemical behaviour in water.
View SolutionWrite the electronic configuration of the element with atomic number 17. Indicate the valency of the element.
View SolutionAn element P(atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reasons :
(a) Write the positions of P and Q in the Modern Periodic Table.
(b) Write the molecular formula of the compound when P reacts with Q.
View Solutionकिसी तत्व A की परमाणु संख्या 12 है और दूसरे तत्व B की परमाणु संख्या 17 है । A और B के संयोग से बने यौगिक का सूत्र लिखें ।
View Solution17परमाणु संख्यावाले तत्वों के इलेक्ट्रॉनिक विन्यास लिखें ।
View Solution20परमाणु संख्यावाले तत्वों के इलेक्ट्रॉनिक विन्यास लिखें ।
View SolutionThe atomic number of an element A is 20 and that of another element B is 17. write down their electronic configuration. Will they produce an electrovalent compound or covalent compound? What will be their valencies? What will be the probable formula of the compound formed between them?
View SolutionConsider two elements ‘A’ (Atomic number 17) and 'B' (Atomic number 19): (i) Write the positions of these elements in the modern periodic table giving justification. (ii) Write the formula of the compound formed when 'A' combines with 'B.' (iii) Draw the electron dot structure of the compound and state the nature of the bond formed between the two elements.
View SolutionAccording to Newlands' law of octaves, the number of elements between two similar elements is
View SolutionAn element P(atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following questions giving reasons :
(a) Write the positions of P and Q in the Modern Periodic Table.
(b) Write the molecular formula of the compound when P reacts with Q.
View Solution(a) List two limitations of Newlands' Law of Octaves.
(b) Write the electronic configuration of two elements A and B whose atomic numbers are 20 and 17 respectively. Write the molecular formula of the compound formed when element A reacts with element B. State whether this compound is acidic, basic or neutral. Give reason to justify your answer.
View Solution
