Choose the correct graph between pressure and volume of ideal gas. 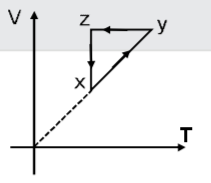
A
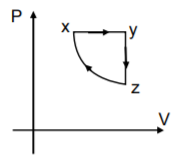
B
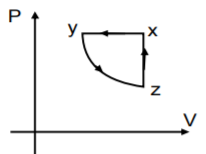
C
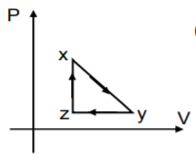
D
वीडियो समाधान
लिखित उत्तर
The correct Answer is:A
NA
|
टॉपर्स ने हल किए ये सवाल
समान प्रश्न
ज्ञान जांच
The product of the pressure and volume of an ideal gas is
Aa constantBapproximately equal to the universal gas constantCdirectly proportional to its temperatureDinversely proportional to its temperatureThe product of the pressure and volume of an ideal gas is
Aa constantBapproximately equal to the universal gas constantCdirectly proportional to its tempDinversely proportional to its tempThe product of pressure and volume of an ideal gas is
Aa constant independent of temperatureBdirectly prouortional to square of its temperatureCdirectly proportional to its temperatureDinversely proportional to its temperature
The graph between pressure P and volume V for a given mass of a gas a fixed temperature T is a ________ .
View SolutionThe internal energy (U) ,pressure (P) and volume V of an ideal gas are related as U=3PV+4 .The gas is
View SolutionFor adiabatic reversible expansion of an ideal gas the expression relating pressure and volume of the gas is -
View SolutionIdentify correct graph between PV and T for an ideal gas of given number of moles:
View SolutionThe internal energy(U) ,pressure (P) and volume (V) of an ideal gas are related as U=3PV+4 .The gas is:
View Solution
