An haloalkane is made to react with excess of alcoholic ammonia to give mainly
A
1∘ amine
B
mixture of 1∘,2∘,3∘ amines
C
micture of 1∘and3∘ amines
D
mixture of 1∘,2∘,3∘ amines and quarternary ammonium salts
Text Solution
The correct Answer is:A
R−X+NH3(Alc.)→ Mixture of 1∘,2∘,3∘ amines and quarternary ammonium salts.
If NH3(alc.) is taken in excess 1∘ amine is the major product.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
An halokane is made to treat with excess of alcholic ammonia to give mainly
A1∘ amineB2∘ amineC1∘,2∘and3∘ aminesDmixture of 1∘,2∘,3∘ amines and quaternary ammonium saltFormaldehyde reacts with excess of ammonia to give
ACH2=NHB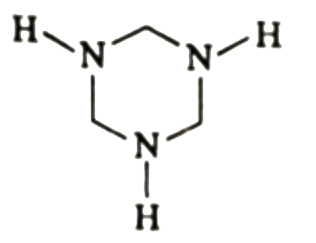 C
C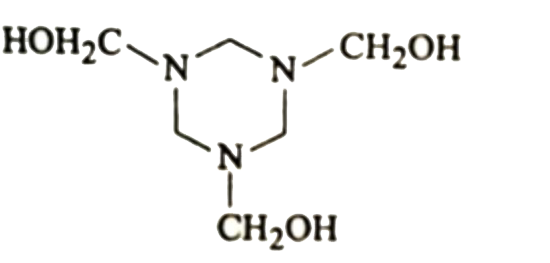 DHexamethylenetetramine
DHexamethylenetetramineAmmonia reacts with excess of chlorine to give
ANH4ClBCl2CNCl3DNCl5
Ethyl bromide reacts with excess of alcoholic ammonia the major product is
View SolutionAniline reacts with excess alkyl halide to give
View SolutionAniline reacts with excess of alkyl halide to give
View SolutionEthyl amine reacts with excess of methyl iodide to give
View SolutionEthyl amine reacts with excess of methyl iodine to give
View Solution

