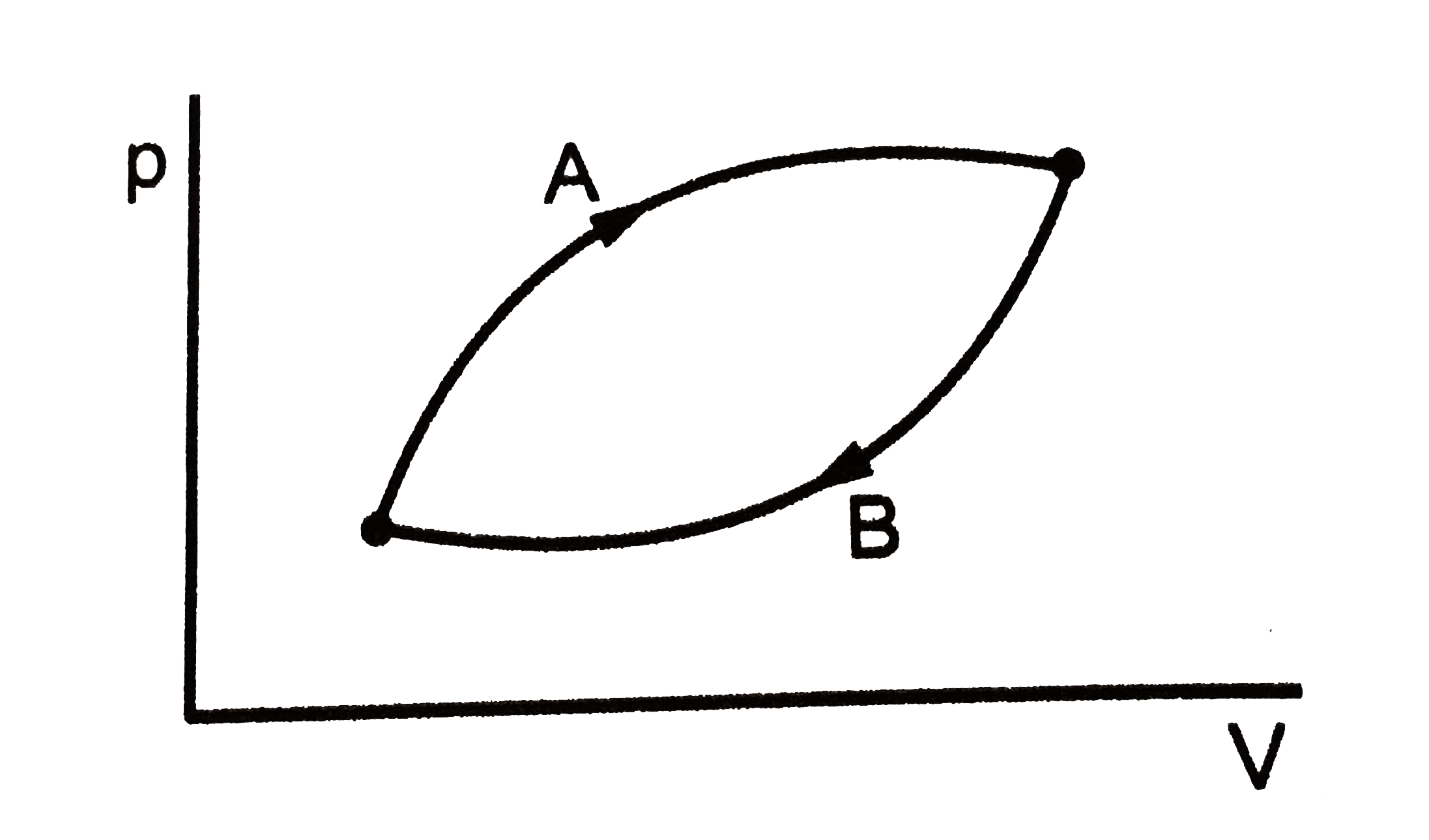
Refer to let ΔU1 and ΔU2 be the changes in internal energy in the system in process A+B and ΔW be the net work done by the system in the process A+B,
A
ΔU1+ΔU2=0.
B
ΔU1−ΔU2=0
C
ΔQ−ΔW=0.
D
ΔQ+ΔW=0.
Video Solution
Text Solution
The correct Answer is:A, C
ΔU1+ΔU2=0.,DeltaQ-DeltaW=0.
|
Topper's Solved these Questions
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Exercises
Explore 22 Videos
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Short Answer
Explore 15 Videos
- LAWS OF THERMODYNAMICSBook:HC VERMAChapter:LAWS OF THERMODYNAMICSExercise:Objective 1
Explore 9 Videos
- KINETIC THEORY OF GASESBook:HC VERMAChapter:KINETIC THEORY OF GASESExercise:Exercises
Explore 62 Videos
- NEWTON'S LAWS OF MOTIONBook:HC VERMAChapter:NEWTON'S LAWS OF MOTIONExercise:Exercises
Explore 42 Videos
Similar Questions
Knowledge Check
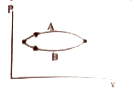
Refer to let ΔU1 and ΔU2 be the changes in internal energy of the system in the processes A and B. then
AΔU1>ΔU2BΔU1=ΔU2CΔU1<ΔU2DΔU1≠ΔU2Let ΔU1andΔU2 be the changes in energy of the system in the process A and B then.
 AΔU1>ΔU2BΔU1=ΔU2CΔU1<ΔU2DΔU1≠ΔU2
AΔU1>ΔU2BΔU1=ΔU2CΔU1<ΔU2DΔU1≠ΔU2In given figure, let ΔU1 and ΔU2 be change in internal energy in process ABC and CDA respectively. ΔQ and W be the net heta given and net work done by the system in the process ABC+CDA, then
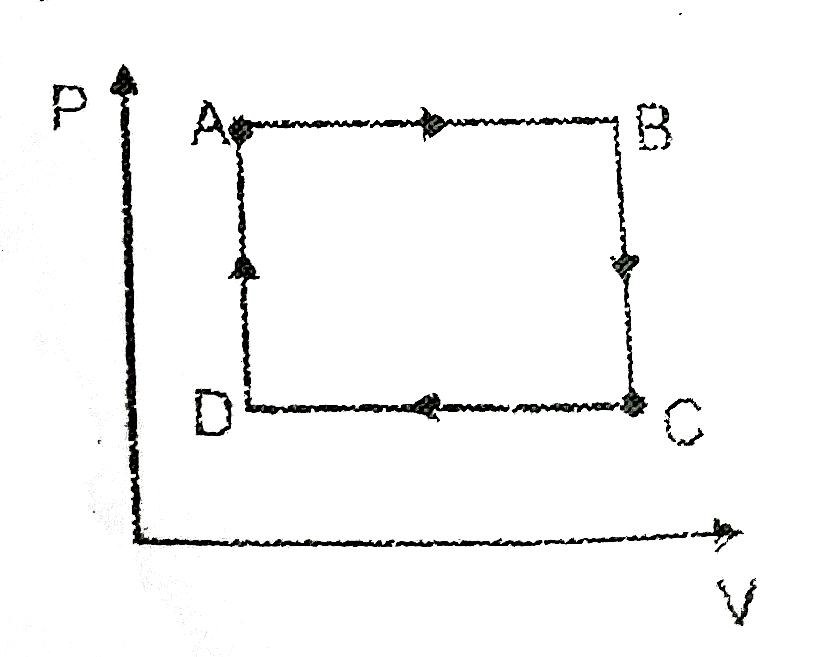 AΔU1+ΔU2=0BΔU1−ΔU2=0CΔQ−W=0DΔQ+W=0
AΔU1+ΔU2=0BΔU1−ΔU2=0CΔQ−W=0DΔQ+W=0
In a cyclic process the change in the internal energy of system is
View SolutionIn a cyclic process, work done by the system is
View SolutionIn a cyclic process, work done by the system is
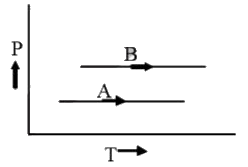
View SolutionConsider the two process on a system as shown in figure. The volumes in the initial state and in the final state are the same in the two process A and B. If W1 and W2 be the work done by the system in the processes A and B respectively then-
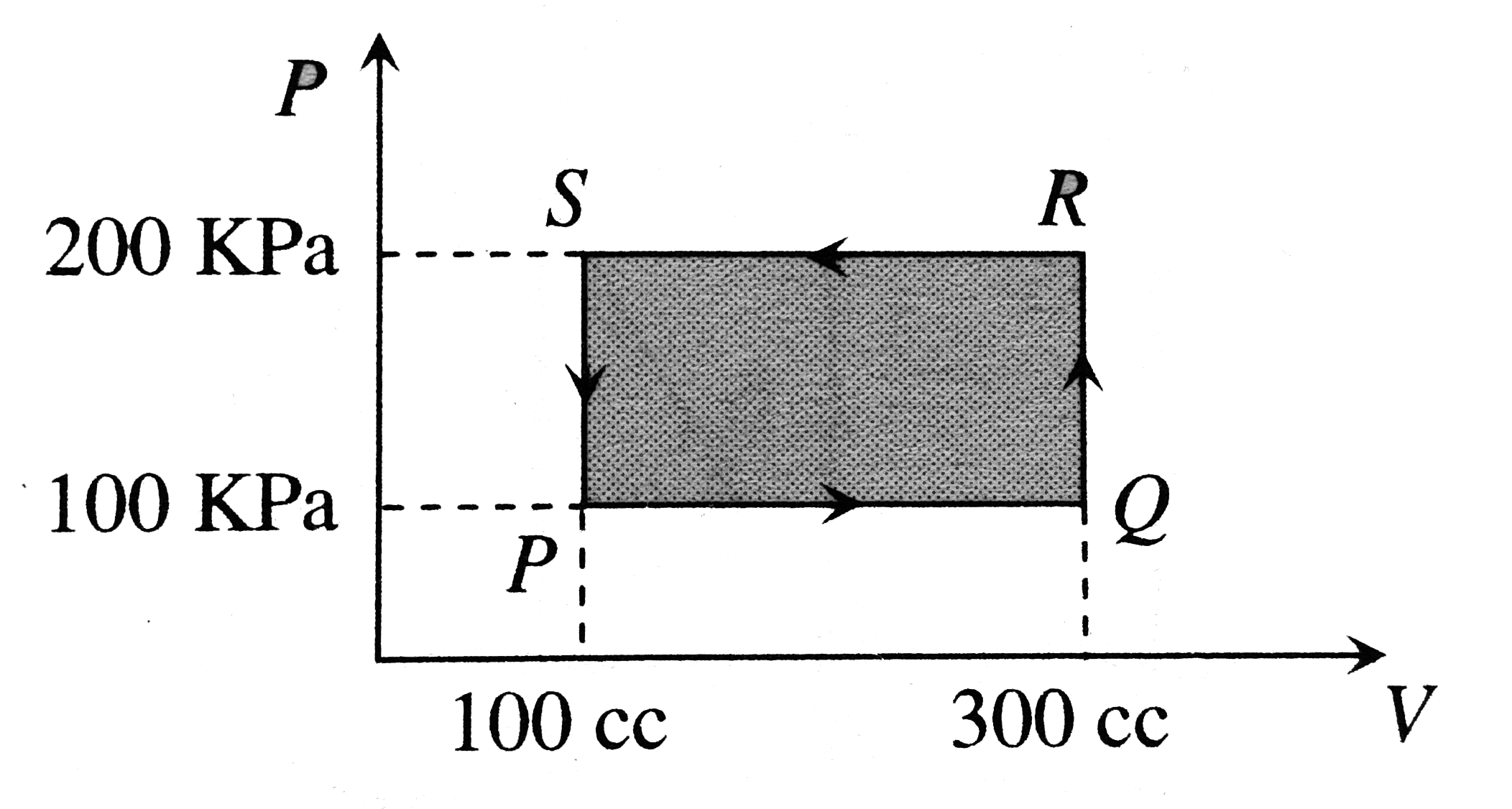
View SolutionA thermodynamic system is taken through the cyclic PQRSP process. The net work done by the system is
View Solution
