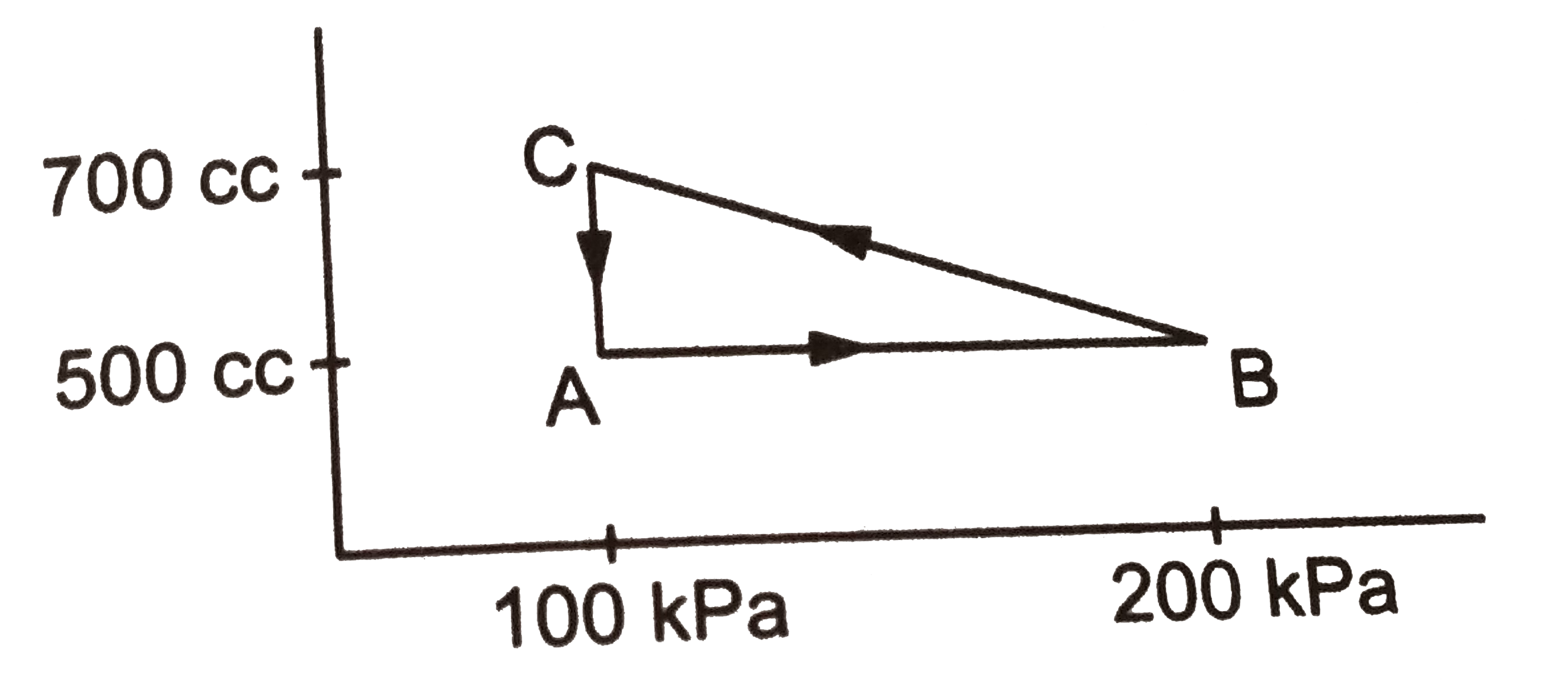
A gas is taken through a cyclic process ABCA as shown in, if 2.4 cal of heat is given in the process, what is the value of J ?
The correct Answer is:A, C, D
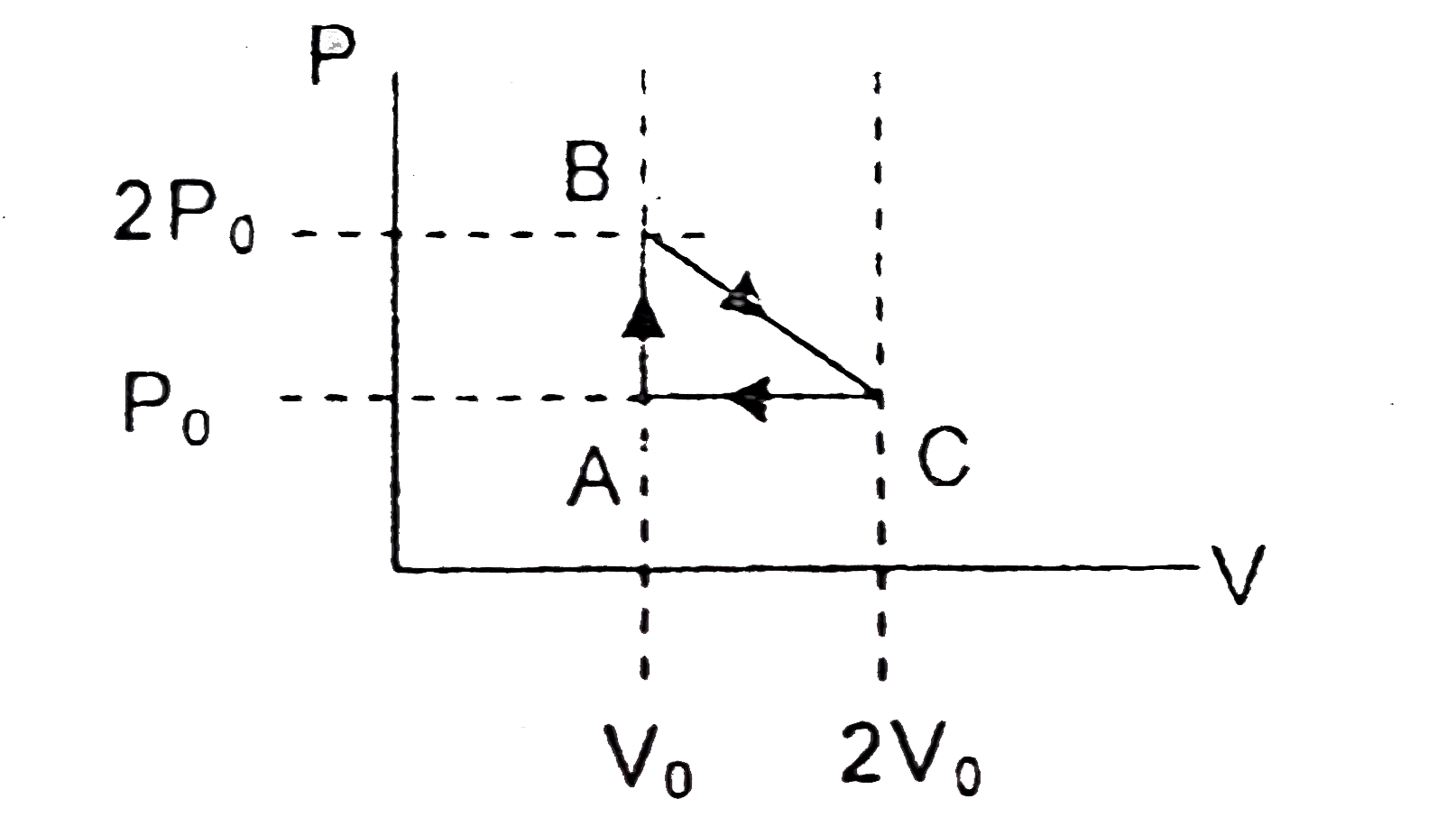
δQ=2.4cal
δW=AB+WBC+WAC
=0+12×(100+200)×103×200xx106(-6)-100xx10^3xx200xx10^(-6)<br>(1)/(2)xx300xx10^3xx200xx10^(-6)-20<br> = 30J = -20J = 10J<br>Delta U = 0(in a cycle process)<br>DeltaQ = Delta U+DeltaW<br>rArr 2.4J = (10)/2.4) = (100)/(24) = (25)/(6) = 4.17J/cal
टॉपर्स ने हल किए ये सवाल
- LAWS OF THERMODYNAMICSBOOK - HC VERMACHAPTER - LAWS OF THERMODYNAMICSEXERCISE - Short Answer
15 Videos
- LAWS OF THERMODYNAMICSBOOK - HC VERMACHAPTER - LAWS OF THERMODYNAMICSEXERCISE - Objective 2
5 Videos
- KINETIC THEORY OF GASESBOOK - HC VERMACHAPTER - KINETIC THEORY OF GASESEXERCISE - Exercises
62 Videos
- NEWTON'S LAWS OF MOTIONBOOK - HC VERMACHAPTER - NEWTON'S LAWS OF MOTIONEXERCISE - Exercises
42 Videos
समान प्रश्न
Should the internal energy of a system necessarily increase if heat is added to it?
View SolutionThe pressure p and volume V of an ideal gas both increase in a process.
View SolutionCalculate the volume of 1 mole of an ideal gas at STP.
View SolutionA block of mass 2 kg placed on a long frictionless horizontal table is pulled horizontally by a constant foerce F. It is found to move 10 m in the first two seconds. Find the magnitude of F.
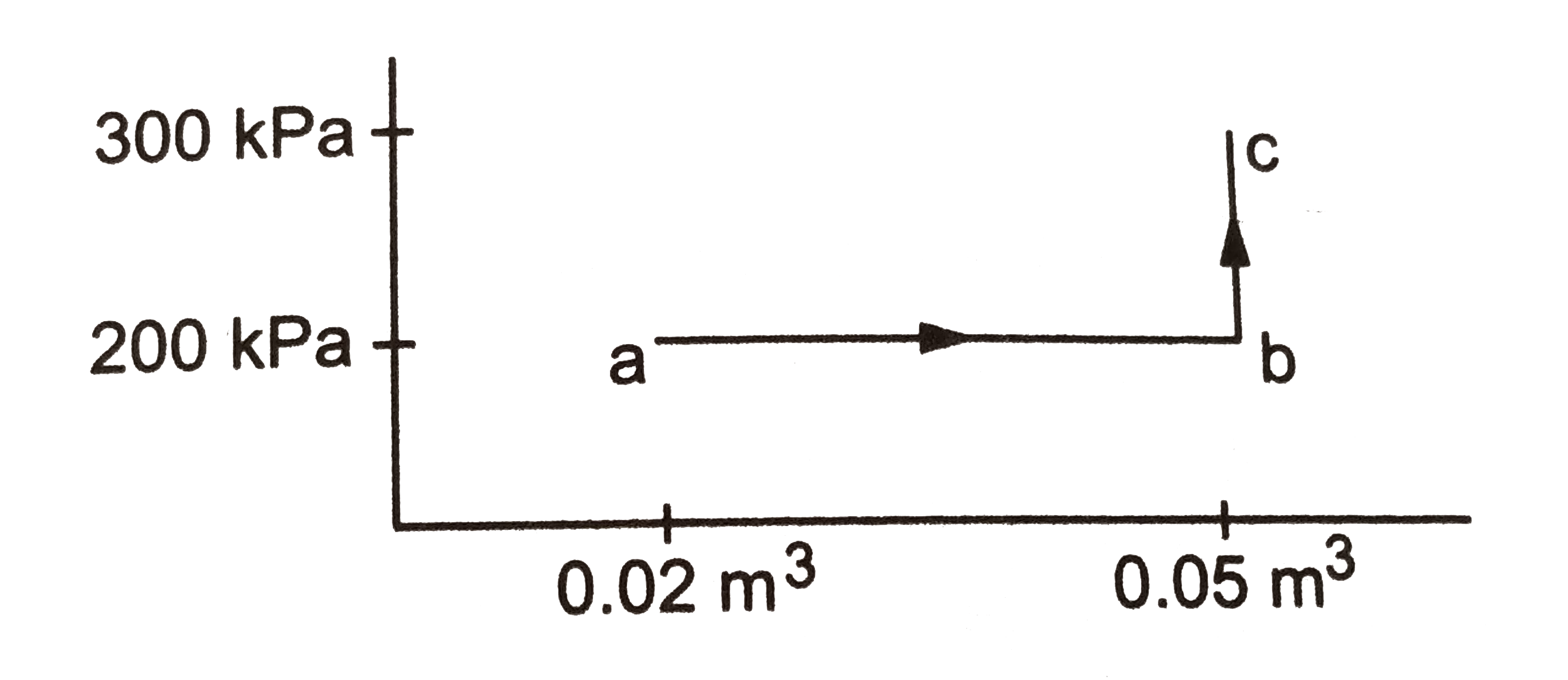
View SolutionA substance is taken through the process abc as shown in, if the internal energy of the substance increase by 5000 J and a heat of 2625 cal is given to the system, calculate the value of J.
View SolutionIn given figure, An ideal gas a gas is taken through a cyclic process ABCA, calculate the value of meachanical equilvalent of heat (J) when 4.8cal of heat is given in the process?
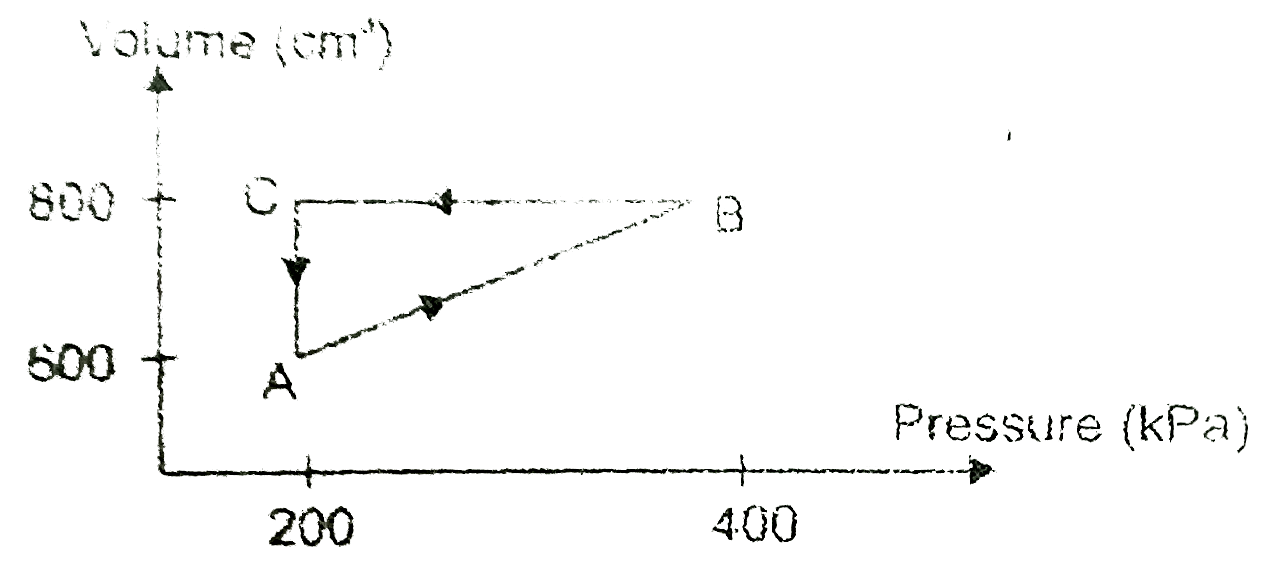
View SolutionA thermodynamics system is taken through the cyclic process abca as shown in the figure. If the heat absorbed by the gas during the cycle is 5x joule then find the value of x.
View SolutionAn ideal monatomic gas undergoes a cyclic process ABCA as shown in the figure. Graph of pressure versus volume of the cyclic process ABCA is
View SolutionA sample of helium is undergoing a cyclic process ABCA as shown. In which of the following processes heat is a absorbed by the gas?
View SolutionOne mole of monoatomic gas taken through a cyclic process as shown in figure.
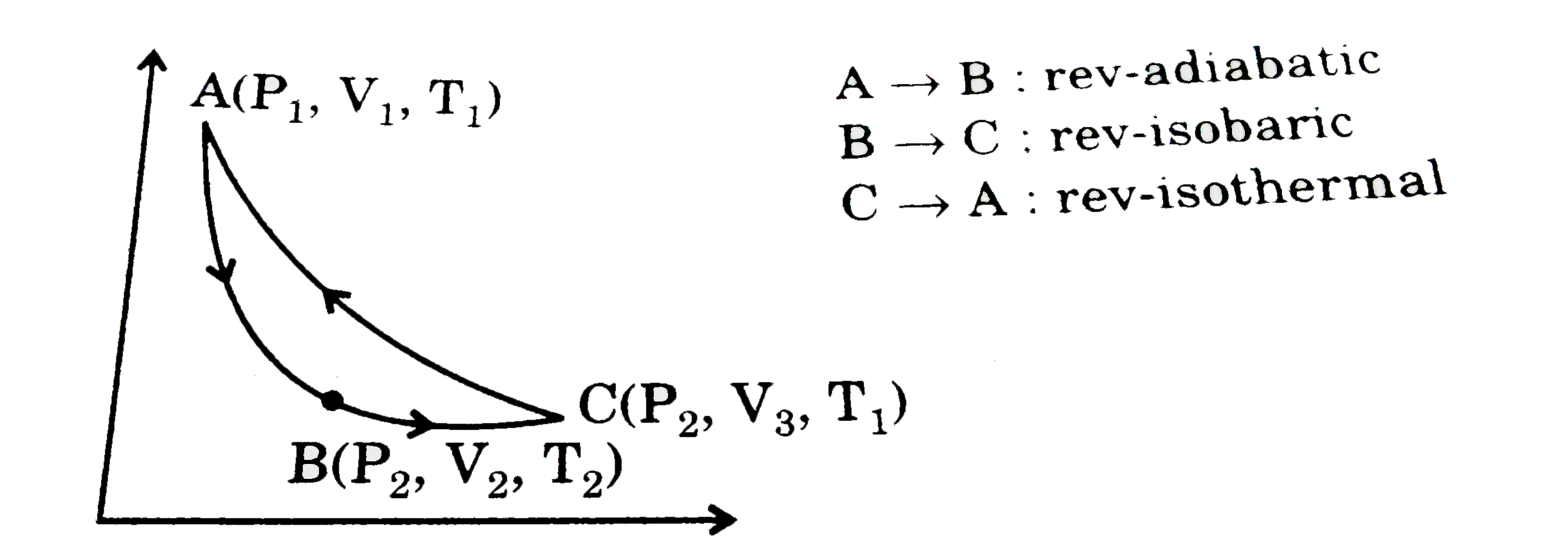
Calculate ∑ABCA=dqrevT.
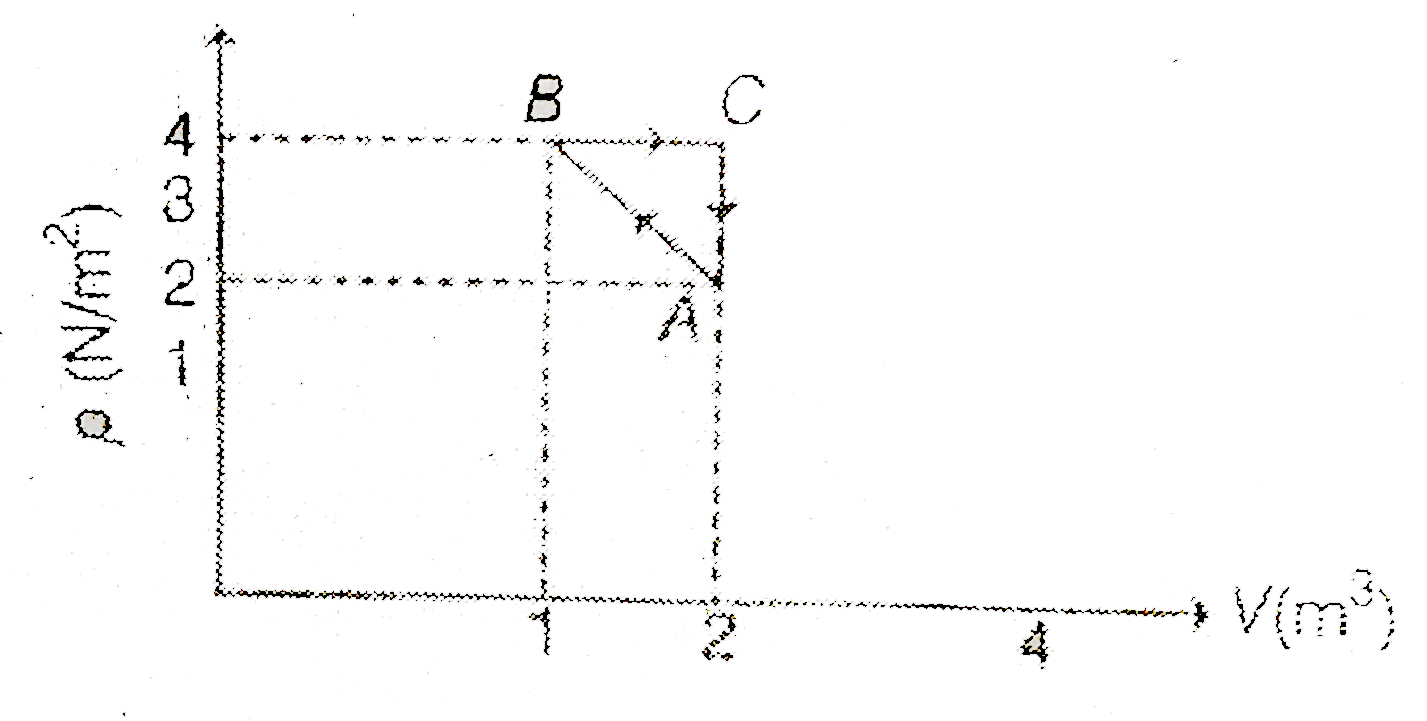
View SolutionCorresponding to the process shown in figure, the heat given to the gas in the process ABCA is (0.2x)J. Find value of x.
View SolutionA gas is taken through a cyclic process ABCA as shown in, if 2.4 cal of heat is given in the process, what is the value of J ?
View Solutionएक आदर्श गैस को चक्रीय प्रक्रम ABCA से गुजारा जाता है चक्र में निहित कार्य होगा
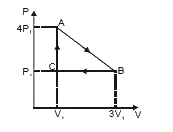
View SolutionCorresponding to the process shown in figure, the heat given to the gas in the process ABCA is (0.2x)J . Find value of x.
View SolutionIn given figure, An ideal gas a gas is taken through a cyclic process ABCA , calculate the value of meachanical equilvalent of heat (J) when 4.8cal of heat is given in the process?
View Solution

