Figure shows a process on a gas in which pressure and volume both change. The molar heat capacity for this process is C.
C=0
C=Cv
C>Cv
C<Cv
The correct Answer is:C
Step by step video, text & image solution for Figure shows a process on a gas in which pressure and volume both change. The molar heat capacity for this process is C. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023टॉपर्स ने हल किए ये सवाल
- SPECIFIC HEAT CAPACITIES OF GASESBook:HC VERMAChapter:SPECIFIC HEAT CAPACITIES OF GASESExercise:Objective 2
Explore 7 Videos
- SPECIFIC HEAT CAPACITIES OF GASESBook:HC VERMAChapter:SPECIFIC HEAT CAPACITIES OF GASESExercise:Exercises
Explore 35 Videos
- SPECIFIC HEAT CAPACITIES OF GASESBook:HC VERMAChapter:SPECIFIC HEAT CAPACITIES OF GASESExercise:Short Answer
Explore 10 Videos
- SOUND WAVESBook:HC VERMAChapter:SOUND WAVESExercise:Exercises
Explore 89 Videos
- THE FORCESBook:HC VERMAChapter:THE FORCESExercise:Exercises
Explore 12 Videos
समान प्रश्न
An ideal gas is taken through a process in which pressure and volume vary as P=kV2. Show that the molar heat capacity of the gas for the process is given by C=Cv+R3.
View Solution600J of heat is added to a monatomic gas in a process in which the gas performs a work of 150J. The molar heat capacity for the process is KR. Then find K.
View Solution
ज्ञान जांच
The molar heat capacity for the process shown in fig. is
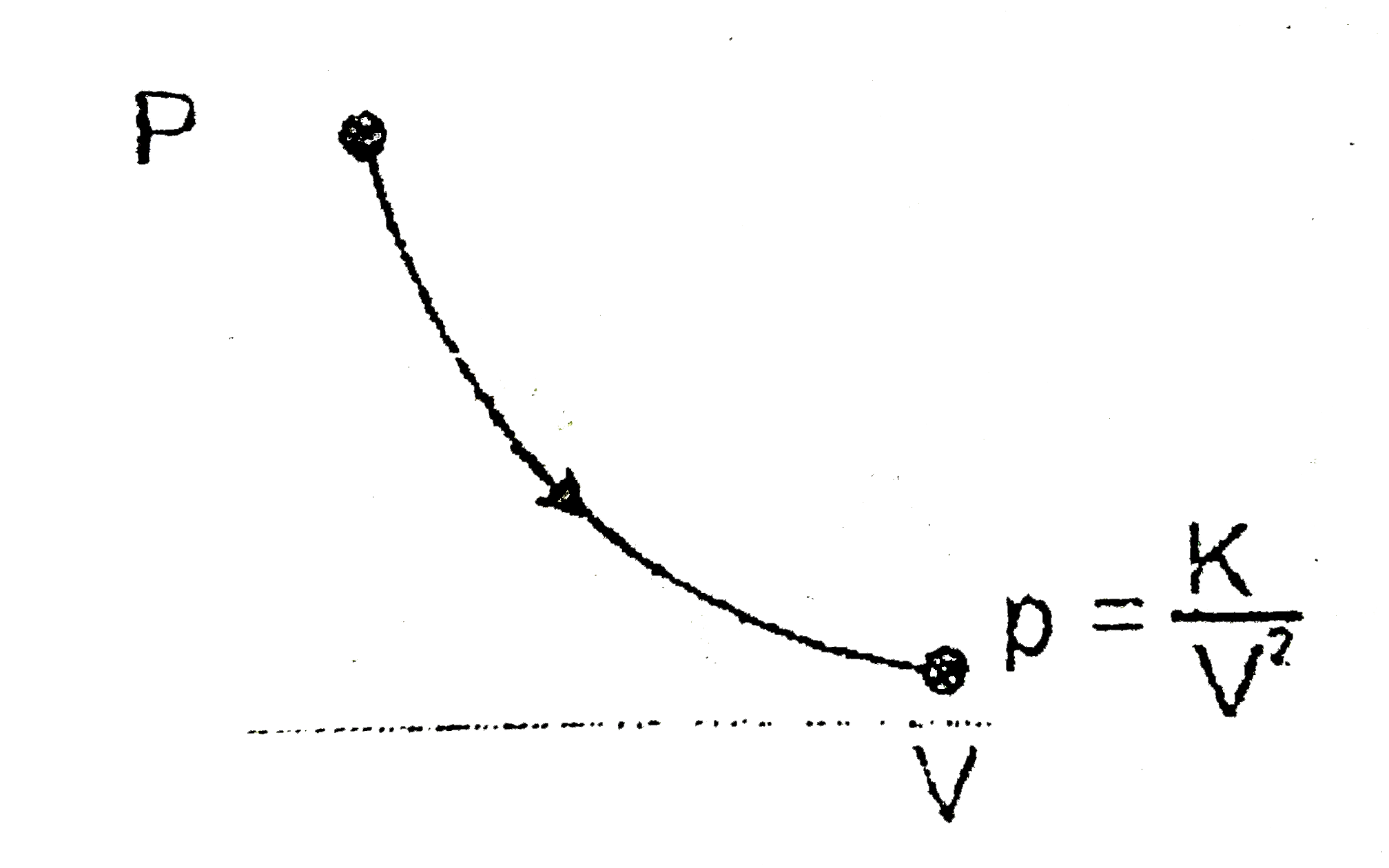 AC=CvBC=CpCC>CvDC<Cv
AC=CvBC=CpCC>CvDC<CvThe molar heat capacity for the process shown in fig. is
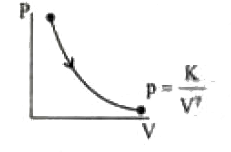 AC=CpBC=CvCC>CvDC=0
AC=CpBC=CvCC>CvDC=0The molar heat capacity of a gas in a process
Ais either CVorCPBlies between CVorCPCmay range from zero to infinityDcan have any real value
The molar heat capacity for the process shown in figure is
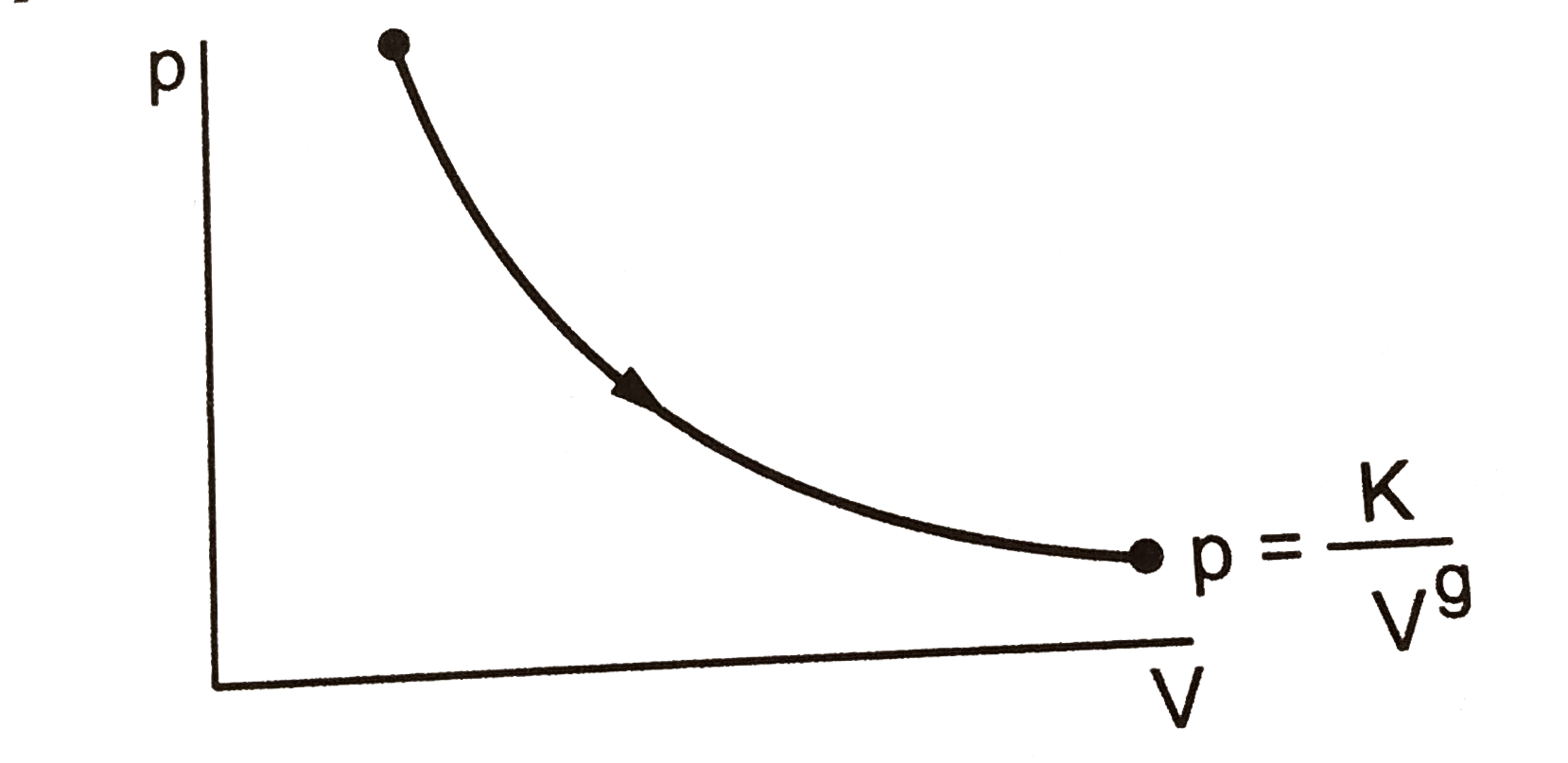
View Solution600J of heat is added to a monoatomic gas in a process in which the gas performs a work of 150J. The molar heat capacity for the process is
View SolutionA diatomic ideal gas is heated at constant volume until the pressure is doubled and again heated at constant pressure until volume is doubled. The average molar heat capacity for whole process is -
View SolutionA diatomic ideal gas is heated at constant at constant volume until the pressure is doubled and again heated of constant pressure until the volume is doubled. The average molar heat capacity for the whole process is
View SolutionIdeal mono-atomic gas is taken through process such that dQ = 3dU. The molar heat capacity for process is:
View Solution
