Let Q and W denote the amount of heat given to an ideal gas the work done by it in an isothermal process.
A
Q=0
B
W=0
C
Q≠W
D
Q=W
Video Solution
Text Solution
The correct Answer is:D
|
Answer
Step by step video, text & image solution for Let Q and W denote the amount of heat given to an ideal gas the work done by it in an isothermal process. by Physics experts to help you in doubts & scoring excellent marks in Class 11 exams.
Updated on:21/07/2023Topper's Solved these Questions
- SPECIFIC HEAT CAPACITIES OF GASESBOOK - HC VERMACHAPTER - SPECIFIC HEAT CAPACITIES OF GASESEXERCISE - Exercises
35 Videos
- SPECIFIC HEAT CAPACITIES OF GASESBOOK - HC VERMACHAPTER - SPECIFIC HEAT CAPACITIES OF GASESEXERCISE - Objective 1
13 Videos
- SOUND WAVESBOOK - HC VERMACHAPTER - SOUND WAVESEXERCISE - Exercises
89 Videos
- THE FORCESBOOK - HC VERMACHAPTER - THE FORCESEXERCISE - Exercises
12 Videos
Similar Questions
Knowledge Check
For an ideal gas, in an isothermal process
AHeat content remains constantBHeat content and temperature remain constantCTemperature remains constantDNone of the aboveIf heat is supplied to an ideal gas in an isothermal process.
Athe internal energy of the gas will increaseBthe gas will do positive workCthe gas will do negative workDthe said process is not possible.If heat is supplied to an ideal gas in an isothermal process.
AThe internal energy of the gas will increaseBThe gas will do positive workCThe gas will do negative workDThe said process is not possible
Work Done In Isothermal Reversible Process
View SolutionThe figures given below show different processes (relating pressure P and volume V) for a given amount of an ideal gas. W is work done by the gas and ΔQ is heat absorbed by the gas.
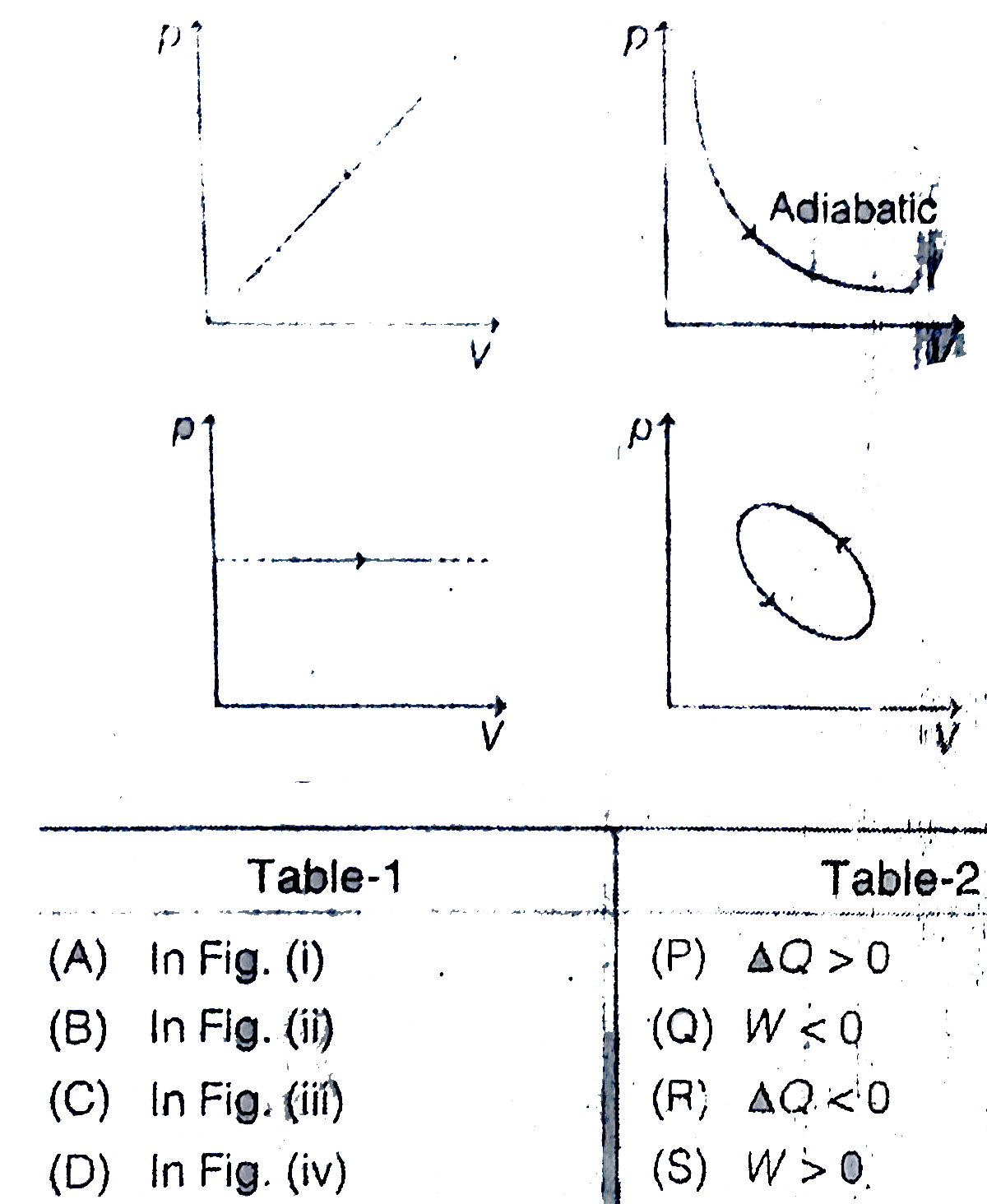
View SolutionCertain amount of heat supplied to an ideal gas under isothermal conditions will result in
View SolutionWhen heat is supplied to an ideal gas in isothermal process, the
View SolutionIn an isothermal process for ideal gas.
View Solution

