An ideal gas (γ=1.67) is taken through the process abc shown in figure . The temperature at the point a is 300k. Calculate (a) the temperature at b and c, (b) the work done in the process, ( c) the amount of heat supplied in the path ab and in the path bc and (d) the change in the internal energy of the gas in the process.
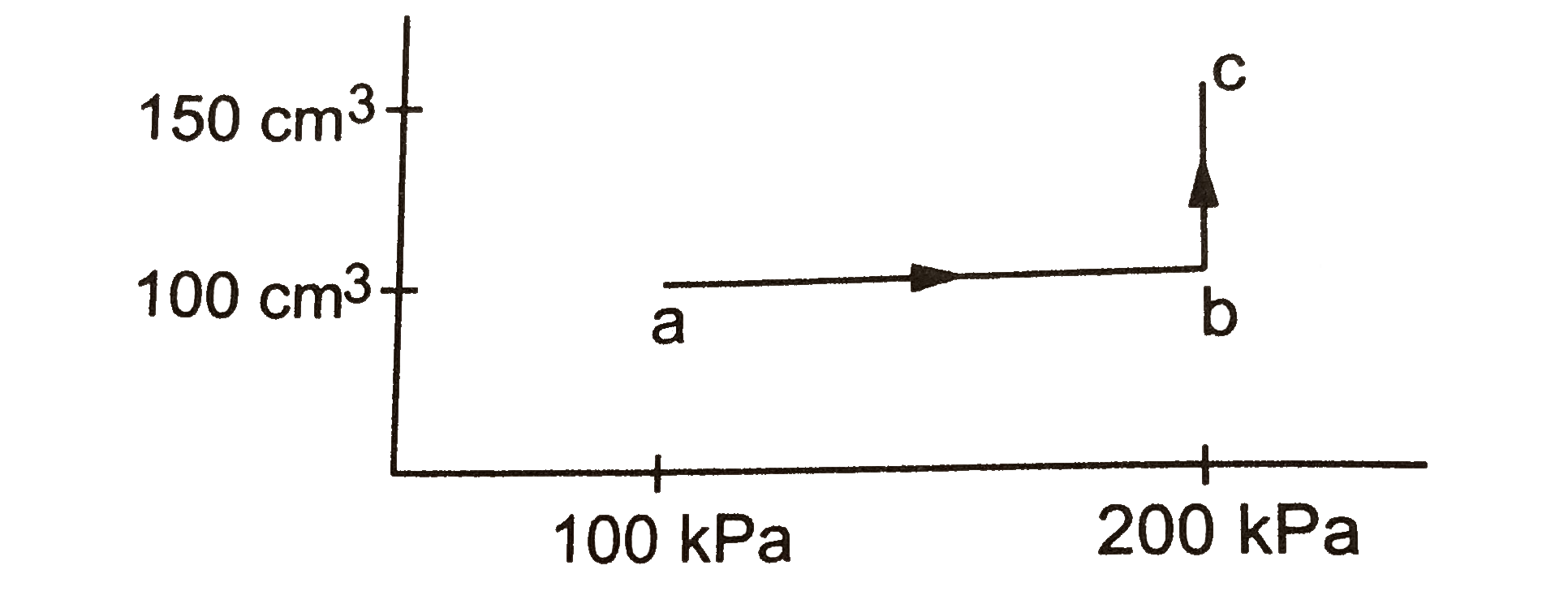
(a) For a, b 'V' is constant
So, P1T1=P2T2
implies 100300=200T2
T2=(200×300100)=600K
For b, c 'p' is constant
So, V1T1=V2T2
implies 100600=150T2
implies T2(600×150100)=900K
(b) Work done = Area enclosed under the graph 50×20KPa
=50×10−6×200×103J
=10J.
(c) 'Q' supplied =nCvdT
Now,
Qbc=(PVRT)×(Rγ−1)×dT
=200×103×100×10−6×300600×0.67<br>=14.925( :. gamma = 1.67)<br>'Q'⊃plied→bc = nCpdT(C_p=(gammaR)/(gamma-1))<br>=(PV)/(RT) xx (gammaR)/(gamma-1) xxdT<br>=(200xx10^(3) xx 150 xx 10^(-6))/(600xx0.67)xx 300<br>=10xx(1.67)/(0.67)=24.925<br>(d)Q= DeltaU+W<br>Now,<br>DeltaU=Q-W<br>=Heat⊃plied−Workdo≠<br>=(24.925 + 14. 925)-10<br>=39.850-10=29.850J.
Topper's Solved these Questions
Similar Questions
Knowledge Check
Ideal gas is taken through process shown in figure
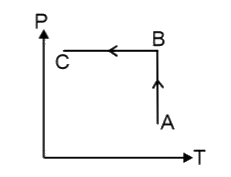 Aln process AB, work done by system is positiveBIn process AB, heat is rejected out of the system.CIn process AB, internal energy increasesDIn process AB internal energy decreases and
Aln process AB, work done by system is positiveBIn process AB, heat is rejected out of the system.CIn process AB, internal energy increasesDIn process AB internal energy decreases and
in process BC internal energy increasesIdeal gas is taken through process shown in figure :
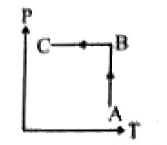 AIn process AB. work done by system is positiveBIn process AB, heat is reacted out of the system.Cprocess AB, internal exergy increasesDIn process AB internal energy decreases and in process BC Internal energy increases.
AIn process AB. work done by system is positiveBIn process AB, heat is reacted out of the system.Cprocess AB, internal exergy increasesDIn process AB internal energy decreases and in process BC Internal energy increases.Ideal gas is taken through the process shown in the figure :
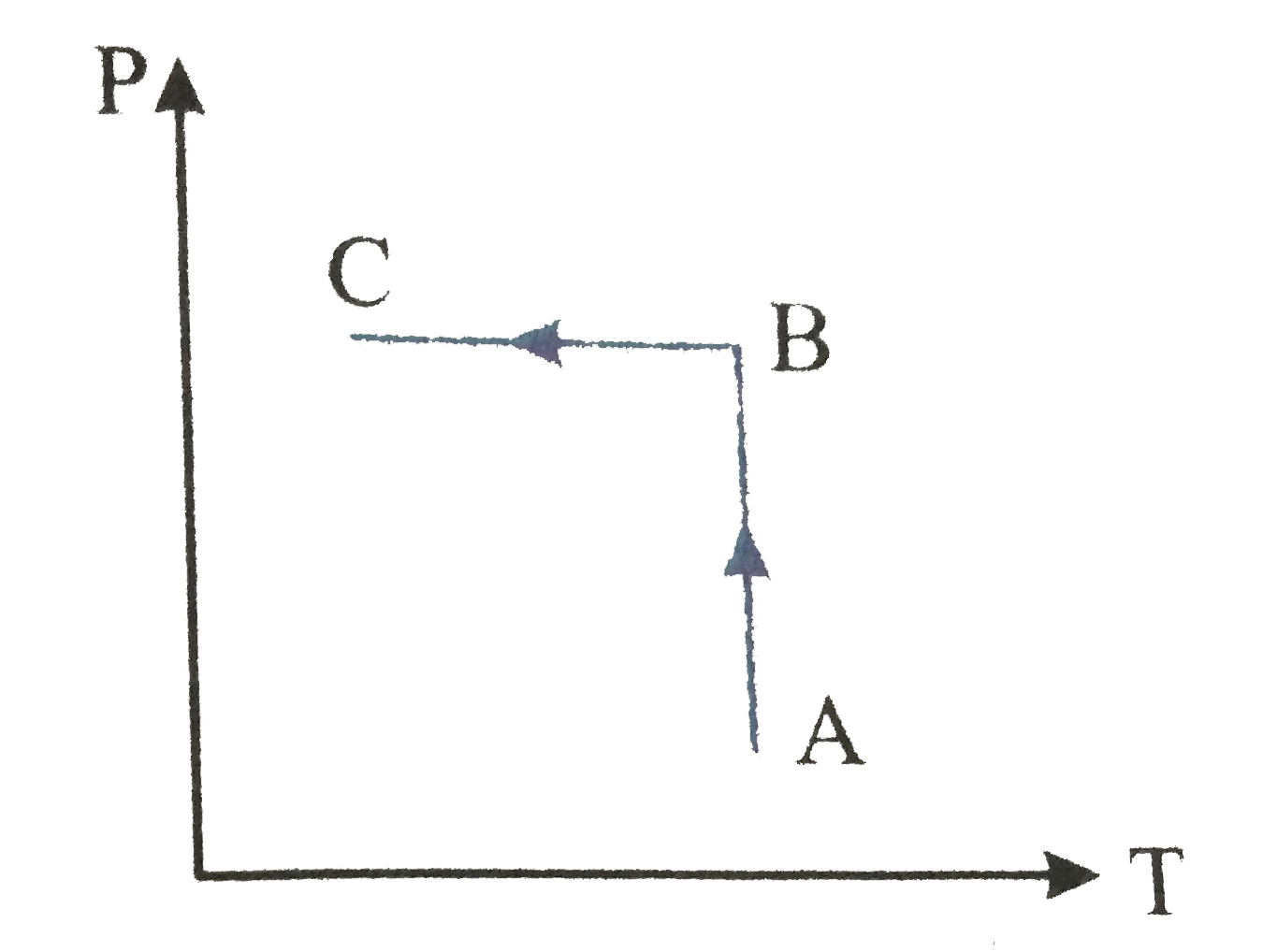 Ain process AB, work done by system is positiveBin process AB, heat is rejectedCin process AB, internal energy increasesDin process AB internal energy decreases and in process BC, internal energy increases.
Ain process AB, work done by system is positiveBin process AB, heat is rejectedCin process AB, internal energy increasesDin process AB internal energy decreases and in process BC, internal energy increases.
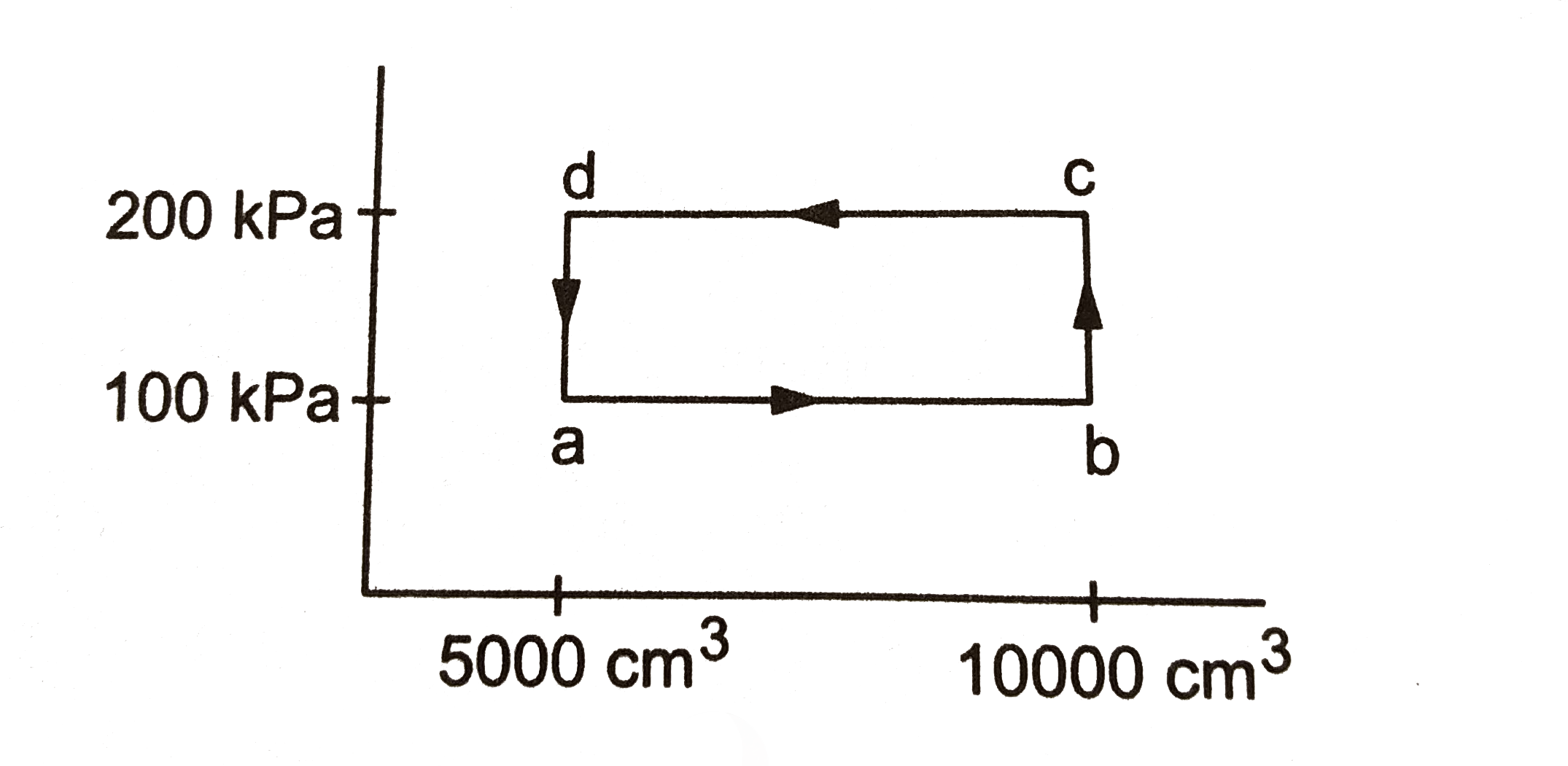
Half mole of an ideal gas (γ=53)istakenthroughthecyc≤abcdaasshown∈figure.Take(R = 25/3 JK^(-1) mol^(-1)).(a)F∈dthetemperatureofthegas∈thestaesa,b,candd.(b)F∈dtheamountofheat⊃plied∈theprocessesabandbc.(c)F∈dtheamountofheatliberated∈theprocessescd and da.
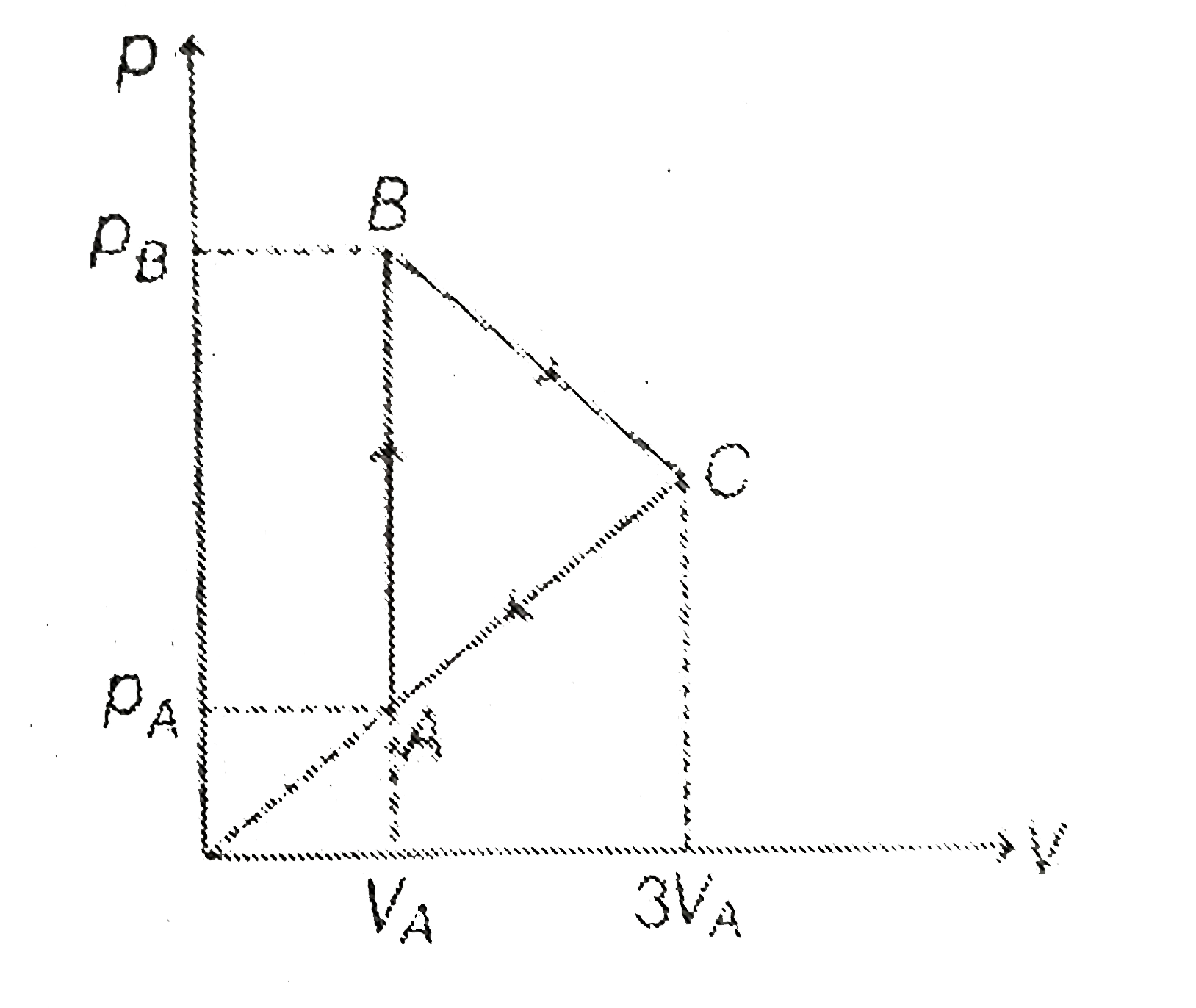
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.
Net work done by the gas in the process is
View SolutionAn ideal gas is taken along the path AB as shown in the figure. The work done by the gas is
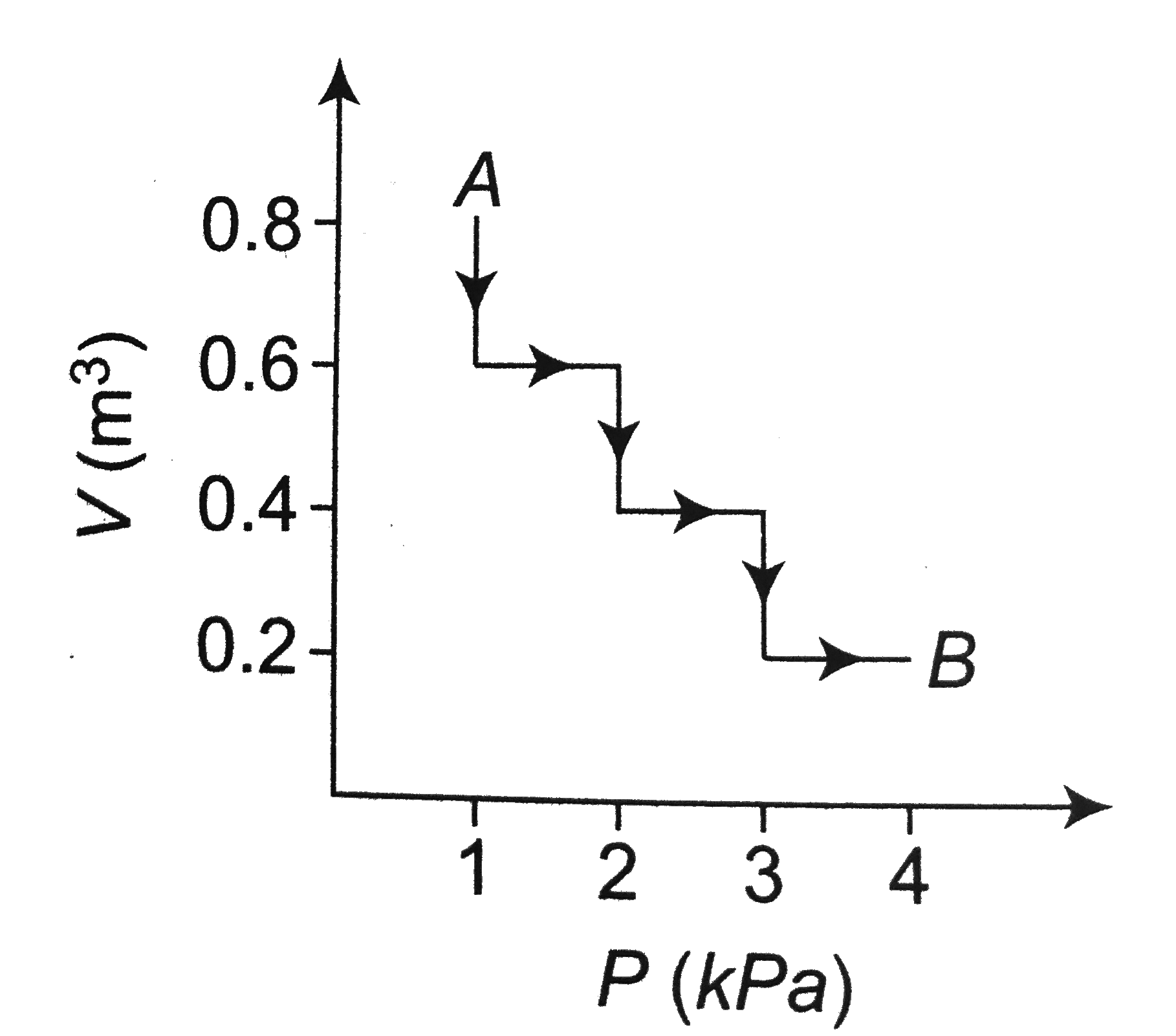
View SolutionA mono-atomic gas is taken along path AB as shown. Calculate change in internal energy of the gas.
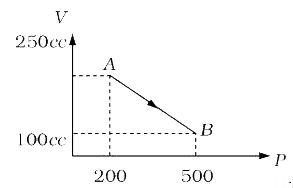
View SolutionA sample of ideal gas is taken through the cyclic process shown in the figure. The temerauture of the gas in state A is TA =200 K. In states B and C the temperature of the gas is the same.

What is the greastes temperature of the gas during the cyclic process ?
View Solution