……….is a polar molecule.
A
N2
B
H2
C
NH3
D
CO2
Text Solution
The correct Answer is:C
|
Topper's Solved these Questions
- MULTIPLE CHOICE QUESTIONSBook:NAVNEET PUBLICATION - MAHARASHTRA BOARDChapter:MULTIPLE CHOICE QUESTIONSExercise:Current Electricity
Explore 5 Videos
- MULTIPLE CHOICE QUESTIONSBook:NAVNEET PUBLICATION - MAHARASHTRA BOARDChapter:MULTIPLE CHOICE QUESTIONSExercise:Magnetic Effect of Electric Current
Explore 4 Videos
- MULTIPLE CHOICE QUESTIONSBook:NAVNEET PUBLICATION - MAHARASHTRA BOARDChapter:MULTIPLE CHOICE QUESTIONSExercise:Interference and Diffraction
Explore 9 Videos
- MODEL QUESTION PAPER FOR PRACTICEBook:NAVNEET PUBLICATION - MAHARASHTRA BOARDChapter:MODEL QUESTION PAPER FOR PRACTICEExercise:SECTION-D
Explore 5 Videos
- QUESTION BANK 2021Book:NAVNEET PUBLICATION - MAHARASHTRA BOARDChapter:QUESTION BANK 2021Exercise:Semiconductors Devices (Long Answer ( LA) ( 4 marks Each) )
Explore 3 Videos
Similar Questions
Knowledge Check
Assertion : CCl4 is a non-polar molecule.
Reason : CCl4 has polar bonds.AIf assertion is true but the reason is falseBIf assertion is false but reason is trueCIF both assertion and reason are true and the reason is the correct explanation of assertionDIf both assertion and reason are true but reason is not the correct explanation of assertionAssertion : CCl4 is a non-polar molecule.
Reason : CCl4 has polar bonds.AIf assertion is true but the reason is falseBIf assertion is false but reason is trueCIF both assertion and reason are true and the reason is the correct explanation of assertionDIf both assertion and reason are true but reason is not the correct explanation of assertionStatement-1 : Allene is a non polar molecule.
Statement-2 : Allene is non planar molecule.AStatement-1 is True, Statement-2 is True, Statement-2 is a correct explanation for Statement-1.BStatement-1 is True, Statement-2 is True, Statement-2 is NOT a correct explanation for Statement-1.CStatement-1 is True, Statement-2 is False.DStatement-1 is False, Statement-2 is True.
Which of the following is a non-polar molecule?
View SolutionWhich one of the following is a non polar molecule?
View SolutionAssertion : C3O2 is non-polar molecule.
Reason : Terminal pi-bonds of the molecule are lying in different planes.
View SolutionAssertion : C3O2 is non-polar molecule.
Reason : Terminal pi-bonds of the molecule are lying in different planes.
View SolutionStatement : p-dimethoxy benzene is polar molecule .
Explanation : The two methoxy gps . At para psositions are located as
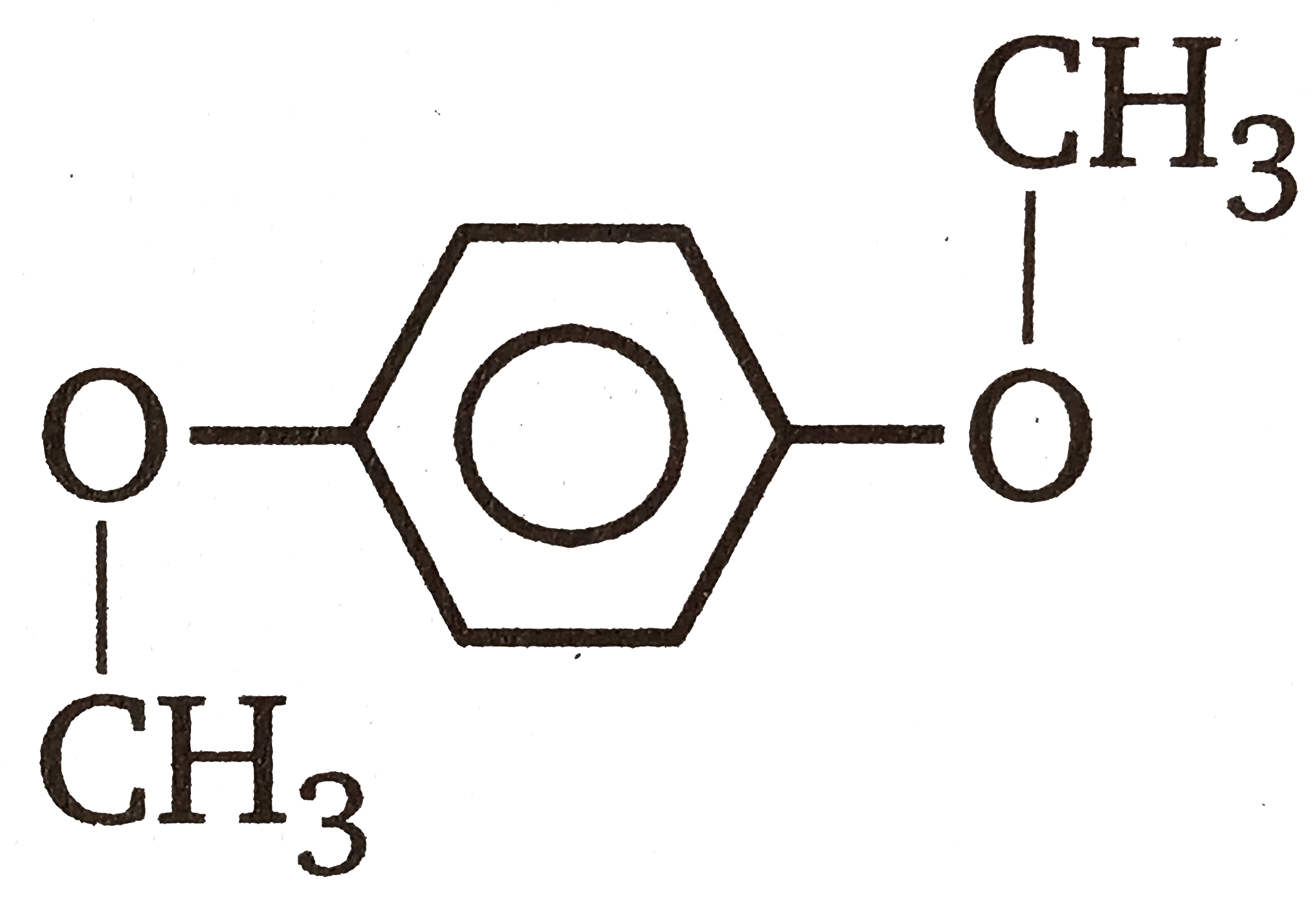
View Solution

