The structure of rhombic sulphur
The following Structure are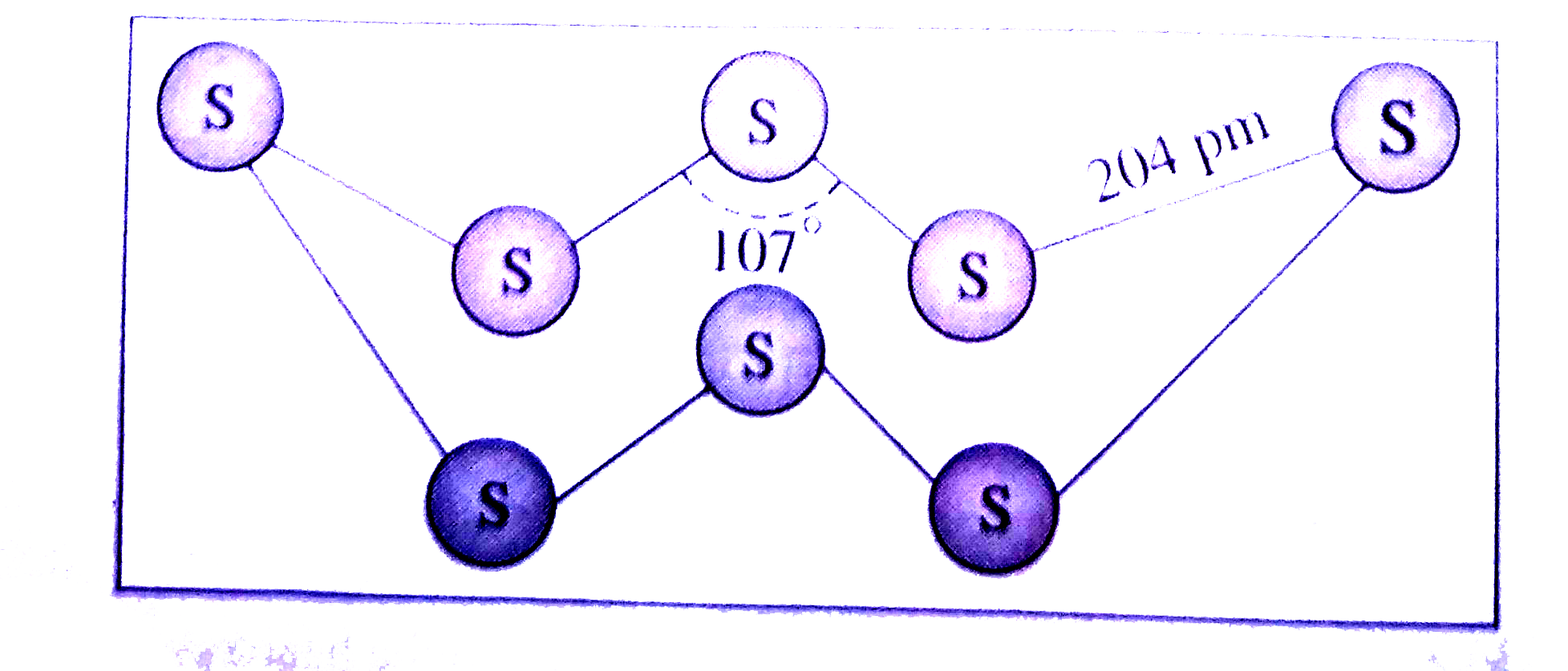
Similar Questions
Knowledge Check
Colour of rhombic sulphur is
AyellowBwhiteCblackDyellowish whiteAlthough the details of the structure of monoclinic sulphur are not well known it probably consists of
AS8 chainsBS2 moleculeCS8 ringsDS4 rings.The incorrect statement for rhombic sulphur is
AIt is insoluble in waterBIt exist as S8 molecules, which is puckered crown ring structureCIt is white in colourDIts melting point is lower than monoclinic sulphur
Sulphur exists is more than one solid form. The stable form at room temperature is rhombic sulphur. But above room temperature the following reaction occurs.
S(rhomic)→S(monoclinic)
If ΔHΘ=276.144Jat298K and 1atm and ΔGΘ=75.312J
a. Calculate ΔSΘ at 298K.
b. Assume that ΔHΘ and ΔSΘ do not vary significantly with temperature, calculate Teq, the temperature at which rhombic and monoclinic sulphur exist in equilibrium with each other.
View SolutionSulphur exists in more than one solid form. The stable form at room temperature is rhombic sulphur. But above room temperature the following reaction occurs :
S (rhombic) → S (monoclinic)
If ΔH=276.144 J at 298 K and 1 atm and ΔG=75.312J
Assume that ΔH and ΔS do not vary significantly with temperature, calculate Teq, the temperature at which rhombic and monoclinic sulphur exist in equilibrium with each other.
View SolutionGive the structure of sulphur tetrafluoride .
View SolutionExplain the structure of sulphur dioxide.
View SolutionI. Melting point of rhombic sulphur is higher than that of monoclinic sulphur.
II. Specific gravity of rhombic sulphur is lower than that of rhombic sulphur.
The incorrect statement(s) is/are
View Solution

