Explain, why aryl halides are less reactive than alkyl halides.
Text Solution
Aryl halides are less reactive tham alkyl halides due to the following reasons :
(1) Resonance effect : In haloarenes, the electron pairs on halogen atom are in conjugation with a-electrons of the benzene ring. The delocalization of these electrons C-Cl bond acquires partial double bond character.
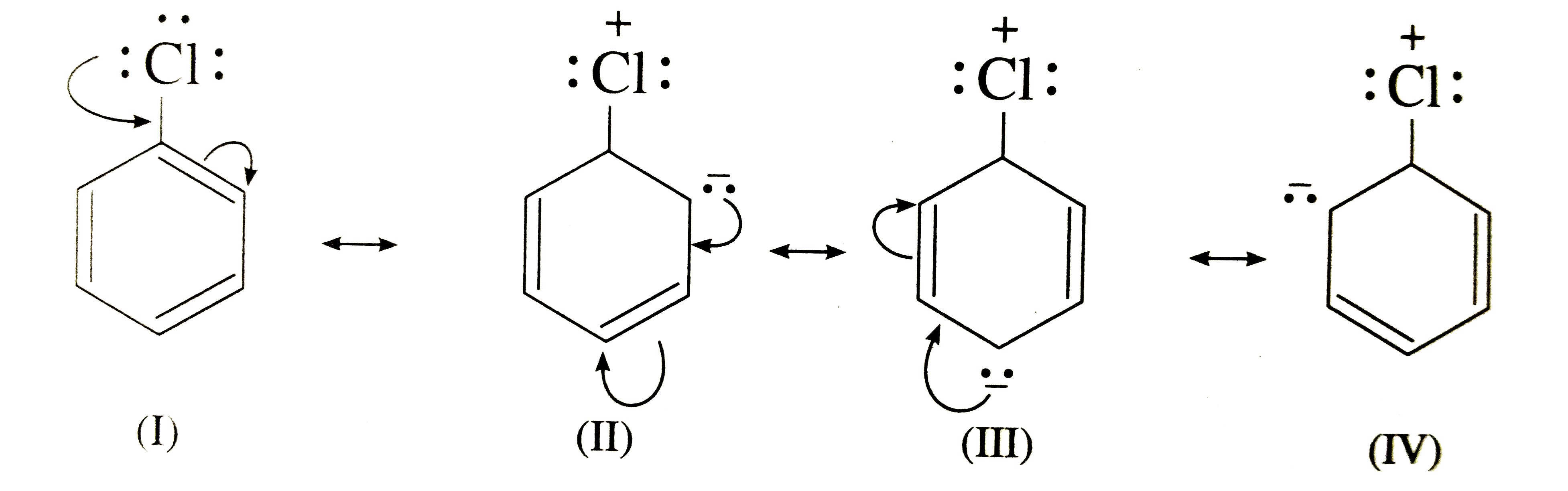
(2) Aryl halides are stabilized by resonance but alkyl halides are not. Hence, the energy of activation for the displacement of halogen from aryl halides is much greater than that of alkyl halides.
Different hybridisation state of carbon atom in C-X bond :
(i) In alkyl halides, the carbon of C-X bond is sp3 hybridized with less s-character and greater bond length of 177 pm, which requires less energy to break the C-X bond.
(ii) In aryl halides, the carbon of C-X bond is sp2 hybridized with more s-character and shorter bond length which requires more energy to break C-X bond. Therefore, aryl halides are less reactive than alkyl halides.
(iii) Polarity of the C-X bond : In aryl halide C-X bond is less polar than in alkyl halides. Because sp?-hybrid carbon of C-X bond has less tendency to release electrons to the halogen than a sp3 -hybrid carbon in alkyl halides. Thus halogen atom in aryl halides cannot be easily displaced by nucleophile.
|
Similar Questions
Knowledge Check
Aryl halides are less reactive than alkyl halides because of
ALonger bond length of C-X than alkyl halidesBShorter bond length of C-X than alkyl halidesCEqual bond length of C - X as that of alkyl halidesDNone of theseAssertion : Alkyl benzene is not prepared by Friedel− Crafts alkylation of benzene.
Reason : Alkyl halides are more reactive than acyl halides.AIf both assertion and reason are true, and reason is the true explanation of the assertion.BIf both assertion and reason are true, but reason is not the true explanation of the assertion.CIf assertion is true, but reason is false.DIf both assertion and reason are falseAssertion : Alkyl benzene is not prepared by Friedel− Crafts alkylation of benzene.
Reason : Alkyl halides are more reactive than acyl halides.AIf both assertion and reason are true and the reason is the correct explanation of the assertionBIf both assertion and reason are true but reason is not the correct explanation of the assertionCif assertion is true but reason is falseDIf assertion and reason both are false
Aryl halides are less reactive than alkyl halides towards nuceophilic reagents. Give reasons.
View SolutionExplain why are aryl halides extremely less reactive towards nucleophilic substitution reactions?
View SolutionAssertion : Alkyl benzene is not prepared by Friedel− Crafts alkylation of benzene.
Reason : Alkyl halides are more reactive than acyl halides.
View SolutionAssertion (A) : Tertiary alkyl halides are more reactive than 1∘ alkyl halides towards elimination.
Reason (R ) : Positive Inductive effect of alkyl groups weakens carbon-halogen bond in 3∘ halides.
View SolutionAssertion (A) Gabriel phthalimide reaction can be used to prepare aryl and alkyl amines.
Reason (R ) Aryl halides have same reactivity as alkyl halides towards nucleophilic substitution reactions.
View Solution

