How is propan-1-0l prepared from (1) propanal (2) propanoic acid?
Text Solution
(1) Propanal: When propanal is hydrogenated at 413K in te presence of finely divided nickel, propan -1-ol isobtained.
CH3Propanal−CH2−CHO+H2Raney Ni−−−−−−−→413KCH3−CH2Propan-1-ol−CH2−OH
(2) Propanoic acid: When propanoic acid is reduced in the presence of LiAlH4 propan-1-ol is formed.
CH3_CH2Propanoic acid−COOH(i)LiAlH4−−−−−−−−→(ii)H3O+CH3−CH2Propan-1-ol−CH2−OH
|
Similar Questions
Knowledge Check
Propan-1-ol can be prepared from propene by
AH2O/H2SO4BHg(OAc)2/H2O followed by NaBH4CB2H6 followed by H2ODCH3CO2H/H2SO4Propan-1-ol may be prepared by the reaction of propene with
AH3BO3BH2SO4 RT/ H2OCB2H6/THF,H2O2 and NaOHD(CH3COO)2Hg/NaBH4Propan1−ol may be prepared by reaction of propene with
ACH3O∣∣C−O−OHBH3BO3CB2H6/NaOH.H2O2DH2SO4/H2O
How can you do the following- conversion?
(a) Pentain-1-ol to Pentanoic acid
(b) Propanal to Propanoic acid ( C) 1-Phenylethanol to 3-Phenylpropanoic acid
( d) Cyclopentene to Pentaanedioic acid
( e) 4-Nitrochlorobenzene to 4-Nitrobenzoic acid
(f)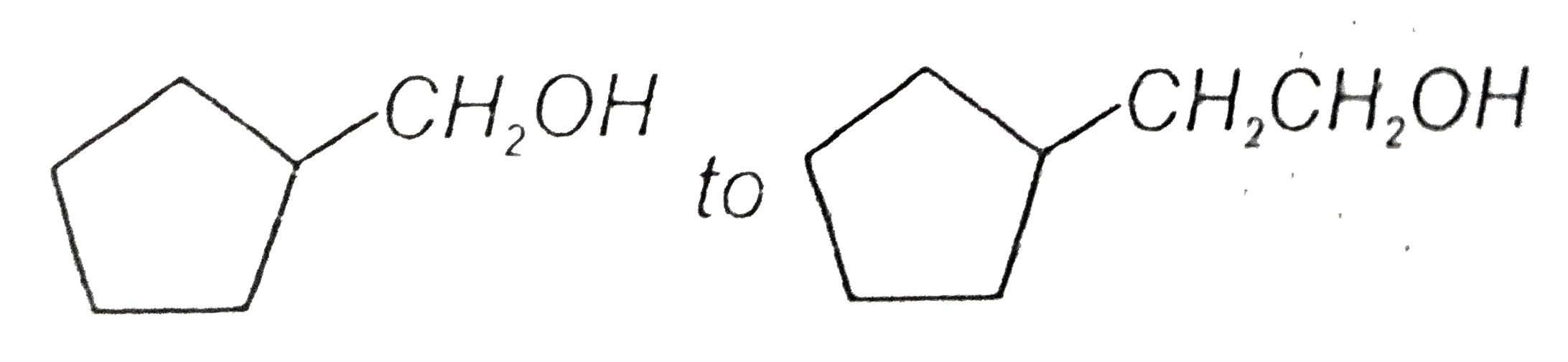
View SolutionHow will you prepare a pure sample of propane ?
View SolutionHow is 1-propoxyproapane synthesised from propan-1-ol ? Write mechanism of the reaction.
View SolutionArrange the following in order of increasing boiling points :
(i) propanone (ii) propanal (iii ) propan -1 ol (iv ) Propane ( v ) Dimethyl ehter
View SolutionHow will you prepare
(i) Acetic acid from acetylene (ii ) butanoic acid from propan -1- ol
(iii ) benzoic acid from aniline (iv ) ethanoic acid from carbon dioxide
View Solution

