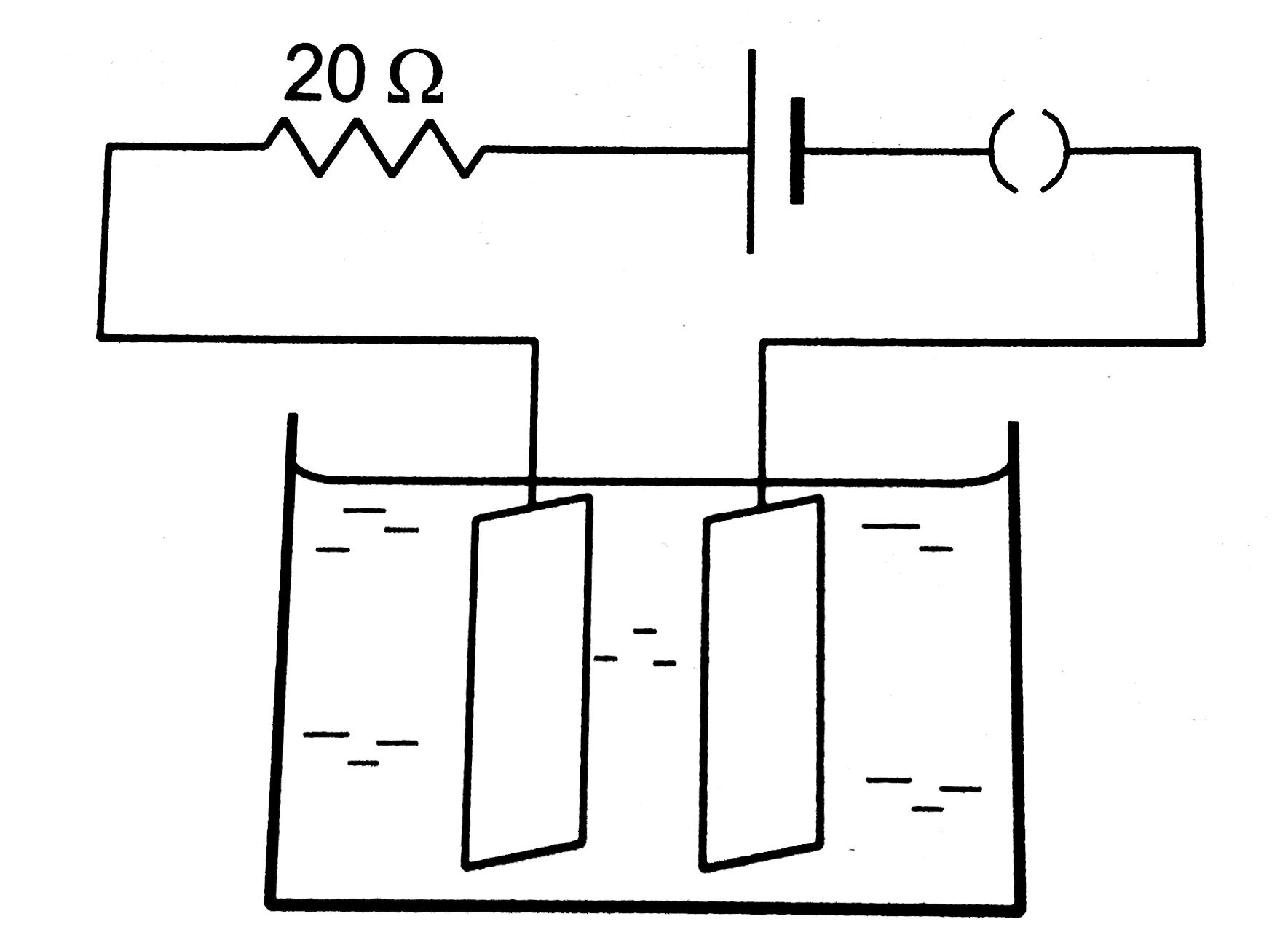
Figure, shows an electrolyte of AgCI through which a current is passed. It is observed that 2.68g of silver is deposited in 10 minutes on the cathode. Find the heat developed in the 20Ω resistor during this period. Atomic weight of silver is 107.9gmol−1. (Figure)
The correct Answer is:A
w=Zit
2.68=107.996500×i×15×60
⇒i=3.99=4amp
.Heatdeveloped∈the20Ωresis→r=(4)2×20×10×60
=16×200×60
=3200×60=192000J=192KJ
Topper's Solved these Questions
- THERMAL AND CHEMICAL EFFECT OF ELECTRIC CURRENTBook:HC VERMAChapter:THERMAL AND CHEMICAL EFFECT OF ELECTRIC CURRENTExercise:Objective 2
Explore 5 Videos
- THE SPECIAL THEORY OF RELATIVITYBook:HC VERMAChapter:THE SPECIAL THEORY OF RELATIVITYExercise:Exercise
Explore 1 Video
- X-RaysBook:HC VERMAChapter:X-RaysExercise:Exercises
Explore 27 Videos
Similar Questions
Two resistors having equal resistances are joined in series and a current is passed through the combination. Neglect any variation in resistance as the temperature changes. In a given time interval,
View SolutionA suitcase kept on a shop's rack is measured50cm×25cm×10cm×bytheshop'sow≠r . A traveller takes this suitcase in a train moving with velocity 0.6c . If the suitcase is placed with its length along the trains velocity , find the dimensions mesured by (a) the traveller and (b)a ground observe .
View SolutionFind the energy, the frequency and the momentum of an X-ray photon of wavelength 0.10 nm.
View SolutionFind the amount of silver liberated at cathode if 0.500A of current is passed through AgNO3 electrolyte for 1 hour. Atomic weight of silver s 107.9gmol−1
View SolutionThe potential difference across the terminals of a battery of emf12V and internal resistance 2Ω drops to 10V when it is connected to a silver voltameter. Find the silver deposited at the cathode in half an hour. Atomic weight of silver is 107.9gmol−1.
View SolutionIf 0.5 amp current is passed through acidified silver nitrate solution for 10 minutes. The mass of silver deposited on cathode, is (eq. wt. of silver nitrate = 108).
View SolutionFind the amount of silver liberated at the cathode if 0.5 A of current is passed through AgNO_(3) electrolyte for 1 hour . Atomic mass of silver = 107.9 g mol^(-1) .
View SolutionAn electric current of 0.4 A is passed through a silver voltameter for half an hour . Find the amount of silver deposited on the cathode. ECE of silver = 1.2×10−3gC−1.
View SolutionHow long will it take to deposit electrolytically 10.79 g of silver on the cathode of a silver voltameter by a current of 25 A ? (ECE of silver= 0.001118gC−1).
View SolutionA current o f1.5 A is passed through a silver voltameter for 35 minutes .The mass of silver deposited during electrolysis is 3.528 g Calculate the electrochmical equivalent of silver.
View SolutionFig .7.2 Shown an eletrolyte AgCl through which current is passes it is observed that 2.68 g of silver is deposited in 10 minutes on the cathode Find the heat devel- oped in the 20Omega resistor during this period .Atomic mass of silver is 107 .9.
View SolutionIf 0.5 amp current is passed through acidified silver nitrate solution for 100 minutes the mass of silver deposited on cathode, is (eq. wt. of silver nitrate=108) :
View SolutionIf 0.5 amp current is passed through acidified silver nitrate solution for 10 minutes. The mass of silver deposited on cathode, is (eq. wt. of silver nitrate = 108).
View SolutionA solution of silver nitrate is electrolysed for 20 minutes with a current of 2 ameres. Calculate the mass of silver deposited at the cathode.
View SolutionFind the amount of silver liberated at cathode if 0.500A of current is passed through AgNO3 electrolyte for 1 hour. Atomic weight of silver s 107.9gmol−1
View SolutionThe potential difference across the terminals of a battery of emf12V and internal resistance 2Ω drops to 10V when it is connected to a silver voltameter. Find the silver deposited at the cathode in half an hour. Atomic weight of silver is 107.9gmol−1.
View Solution
