Which of the following curve may represent the speed of the electron in a hydrogen atom as a function of the principal quantum number n?
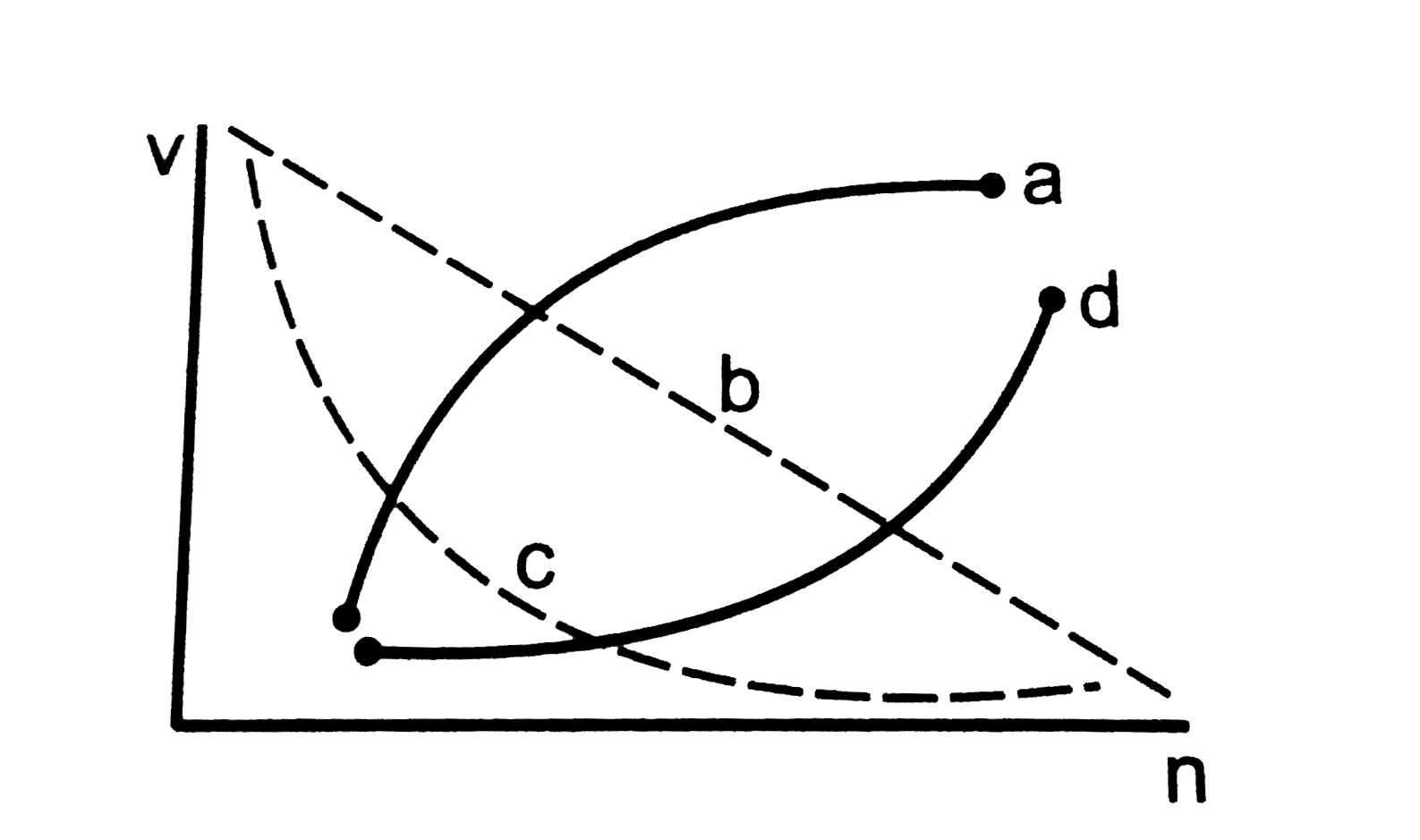
a
b
c
d
The correct Answer is:C
Step by step video, text & image solution for Which of the following curve may represent the speed of the electron in a hydrogen atom as a function of the principal quantum number n? by Physics experts to help you in doubts & scoring excellent marks in Class 12 exams.
Updated on:21/07/2023Topper's Solved these Questions
- BOHR'S MODEL AND PHYSICS OF THE ATOMBook:HC VERMAChapter:BOHR'S MODEL AND PHYSICS OF THE ATOMExercise:Objective 2
Explore 6 Videos
- BOHR'S MODEL AND PHYSICS OF THE ATOMBook:HC VERMAChapter:BOHR'S MODEL AND PHYSICS OF THE ATOMExercise:Exercises
Explore 46 Videos
- BOHR'S MODEL AND PHYSICS OF THE ATOMBook:HC VERMAChapter:BOHR'S MODEL AND PHYSICS OF THE ATOMExercise:Short Answer
Explore 10 Videos
- ALTERNATING CURRENTBook:HC VERMAChapter:ALTERNATING CURRENTExercise:Exercises
Explore 19 Videos
- CAPACITORSBook:HC VERMAChapter:CAPACITORSExercise:Exercise
Explore 2 Videos
Similar Questions
Which ofthe following curves may represent the energy of electron in hydrogen atom as a function of principal quantum number n:
View SolutionWhich of the plots shown in the figure represents speed (vn) of the electron in a hydrogen atom as a function of the principal quantum number (n)?
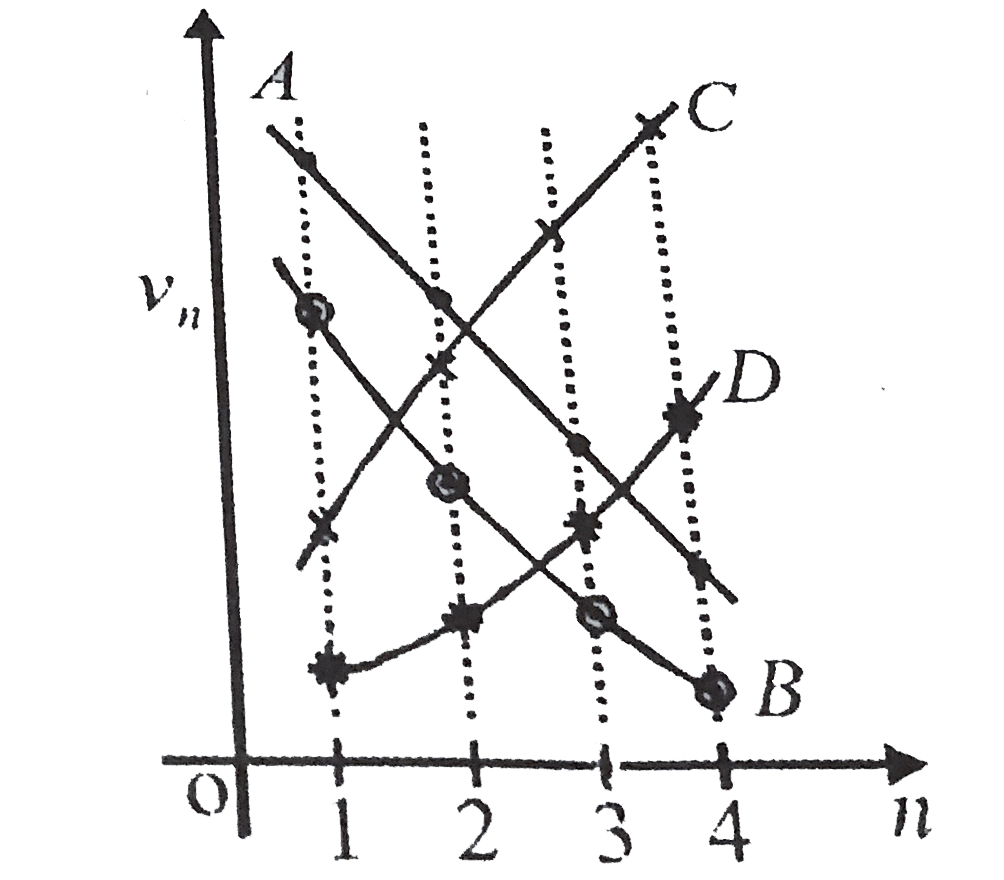
View Solution
Knowledge Check
Which of the following curves may represent the speed of the electron in a hydrogen atom as a function of the principal quantum number n ?
A B
B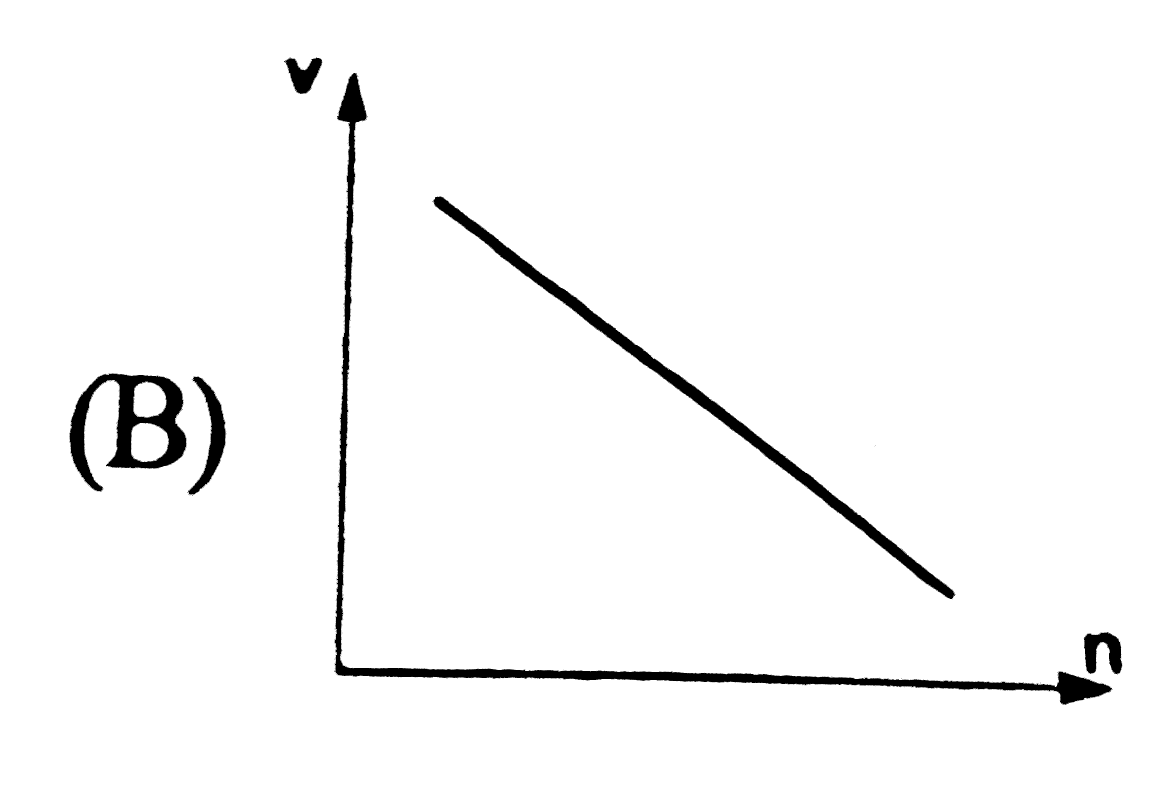 C
C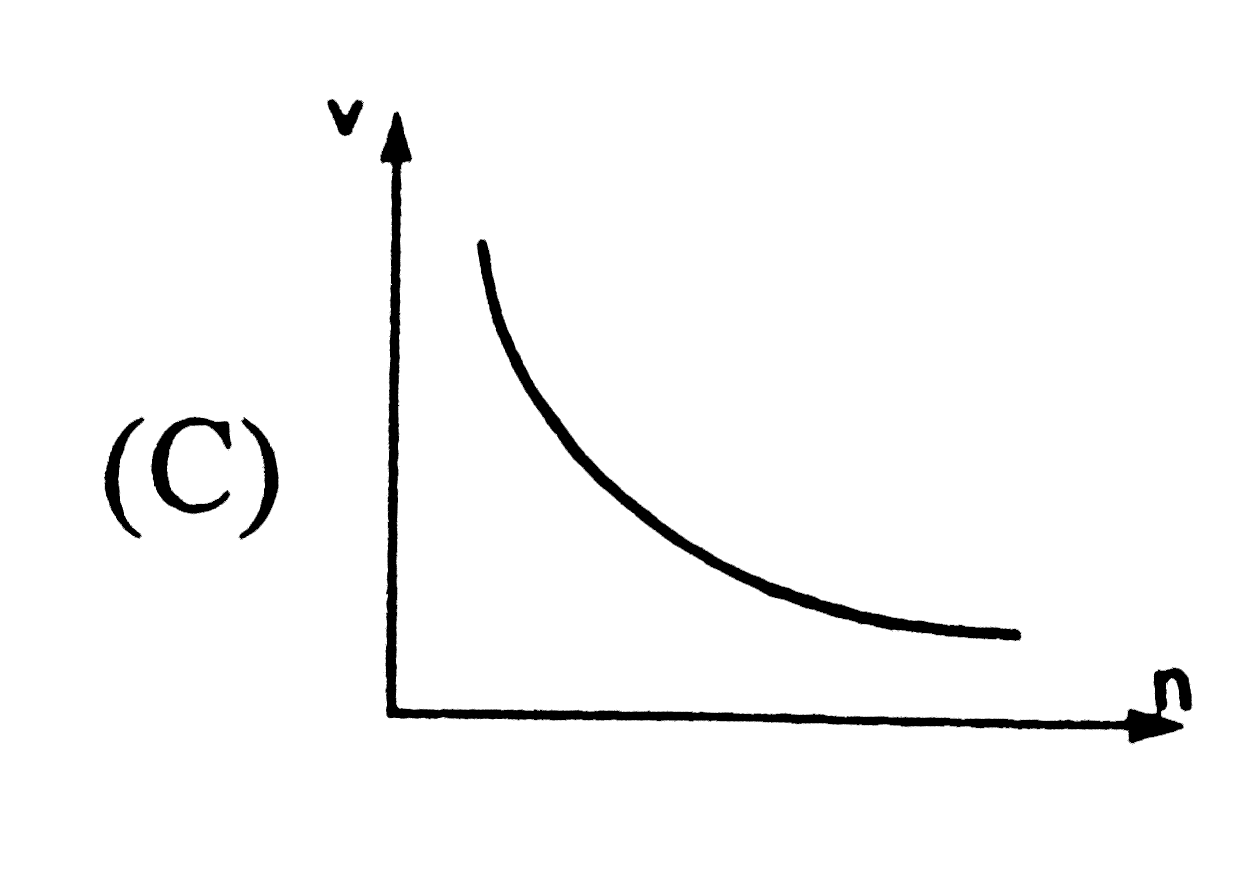 D
D
Which of the following curves may represent the radius of orbit (rn) in a H-atoms as a function of principal quantum number(n)
A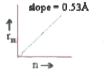 B
B C
C DNone of these
DNone of theseWhich of the following curves may represent the radius of orbit (rn) in H-atoms as a function of principal quantum number (n)
A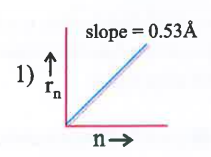 B
B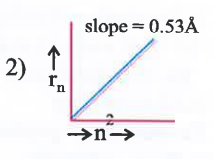 C
C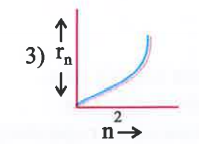 DNone of these
DNone of these
The principal quantum number represent
View SolutionThe force acting on the electron in a hydrogen atom depends on the principal quantum number as
View SolutionThe principal quantum number, n describes
View SolutionThe de Broglie wavelength of the electron in the hydrogen atom is proportional to [n is the principal quantum number]
View SolutionSpeed of a revolving electron around the nucleus varies with principal quantum number n as :
View Solution
