Although F has high -I effect, yet F3C+ is more stable 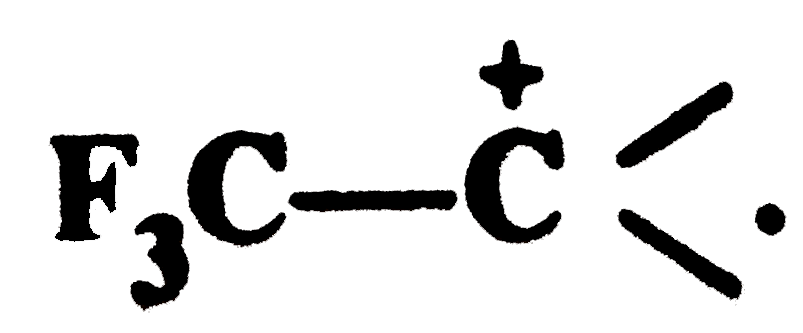 . Explain why is it so ?
. Explain why is it so ?
In carbocation, 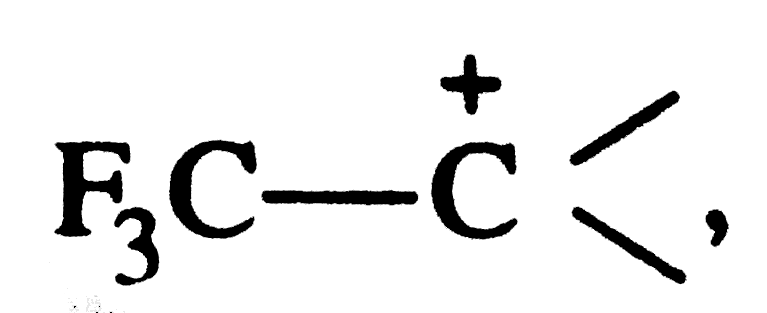 , the strongly electron withdrawing Fe3C− group withdraws electrons of the C-C bond towards itself thereby intensifying the +ve charge and thus destabilising the carbocation.
, the strongly electron withdrawing Fe3C− group withdraws electrons of the C-C bond towards itself thereby intensifying the +ve charge and thus destabilising the carbocation.
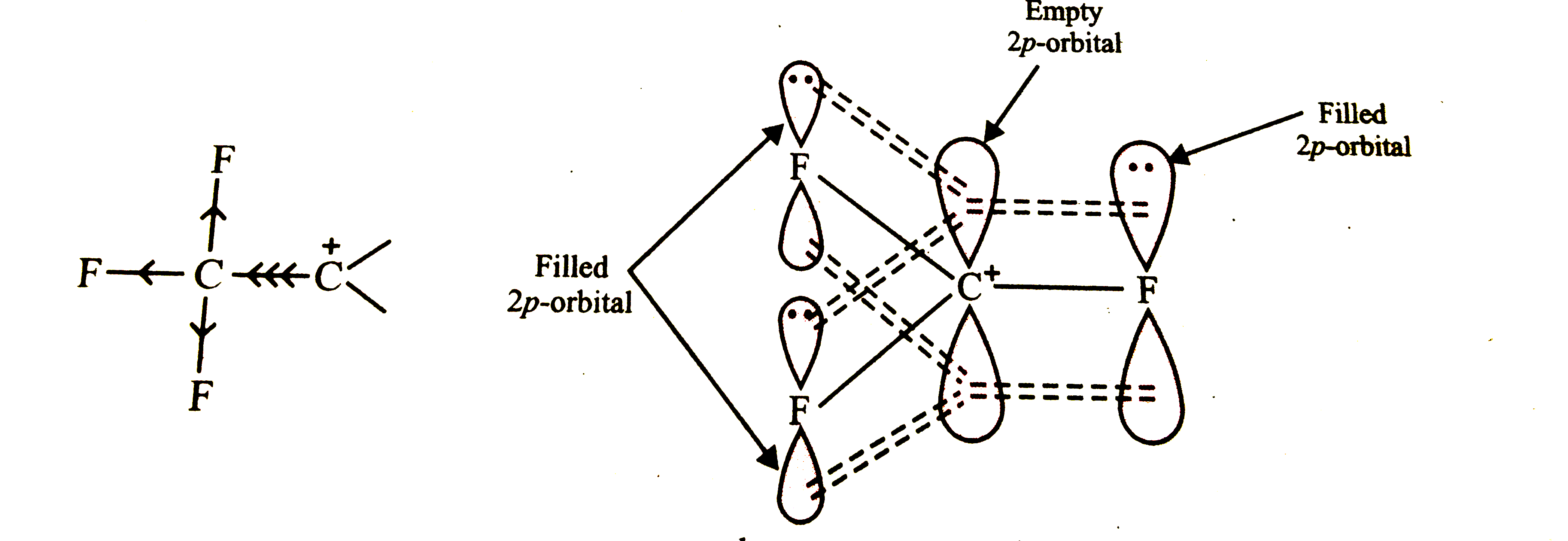
On the other hand, in carbocation F3C+, the lone pair of electrons on each of the three F-atoms overlap with the empty 2p-orbital of the carbon atom carrying the +ve charge thereby dispersing the +ve charge and thus stabilizing the carbon, F3C+ relative to 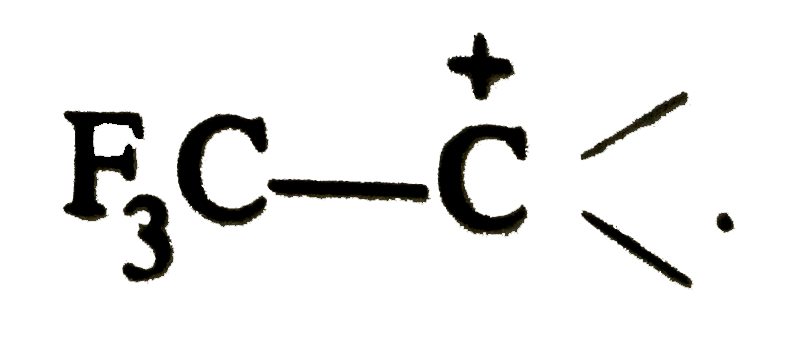 .
.
Topper's Solved these Questions
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Analytical Questions and Problems with Answers/Solutions (Problems)
4 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Competition Focus (Jee (Main and Advanced)/Medical Entrance) I. Multiple choice Questions (With One Correct Answer)
108 Videos
- ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESBOOK - PRADEEPCHAPTER - ORGANIC CHEMISTRY-SOME BASIC PRINCIPLES TECHNIQUESEXERCISE - Additional Questions (Long Answer Type Questions)
10 Videos
- HYDROGEN BOOK - PRADEEPCHAPTER - HYDROGEN EXERCISE - COMPETITION FOCUS (Assertion-Reason Type Questions) Type 2
15 Videos
- P-BLOCK ELEMENTS (NITROGEN FAMILY)BOOK - PRADEEPCHAPTER - P-BLOCK ELEMENTS (NITROGEN FAMILY)EXERCISE - Competition focus jee(main and advanced)/ medical entrance special) (VIII. Assertion-Reason Type Questions)
10 Videos
Similar Questions
Although C-D bond is stronger than C-H bond, yet (CH3)3C+ (i) is more stable than (CD3)3C+ (ii) Why so ?
View SolutionThe carboncation
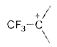 is unstable whereas carbocation F3+C is more stable. Explain
is unstable whereas carbocation F3+C is more stable. Explain
View SolutionWhy is (CH3)3C+ more stable than CH3CH+2 ?
View SolutionExplain the following :
(a) Why MeNH⊕CH2 (I) is more stable more than MeCH2⊕CH2 (II) when both are 1∘ carbocations ?
(b) Which of the following intermediate is unstable ?
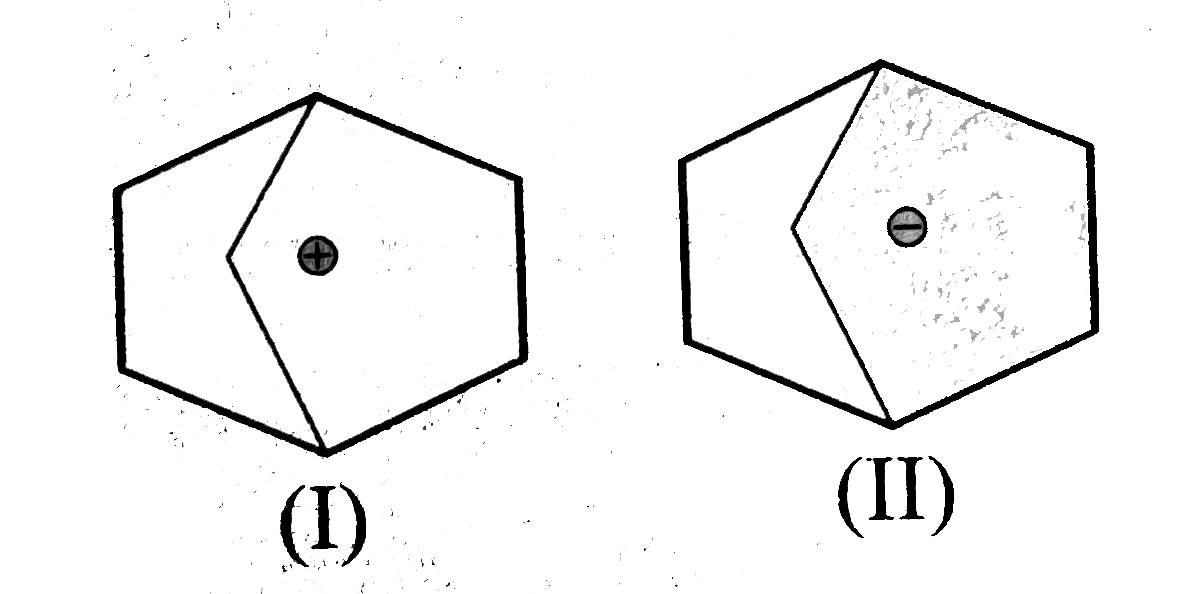
( c) Why allylic free radical (CH2−CH=CH2)(I) is more stable than propy1 free radical (.CH2−CH2−CH3)(II), when both are 1∘ free radicals ?
(d) Arrange the following in the decreasing order of their stabilities :

( e) Which of the following resonance structures of p−nitro phenoxide is not feasible ?
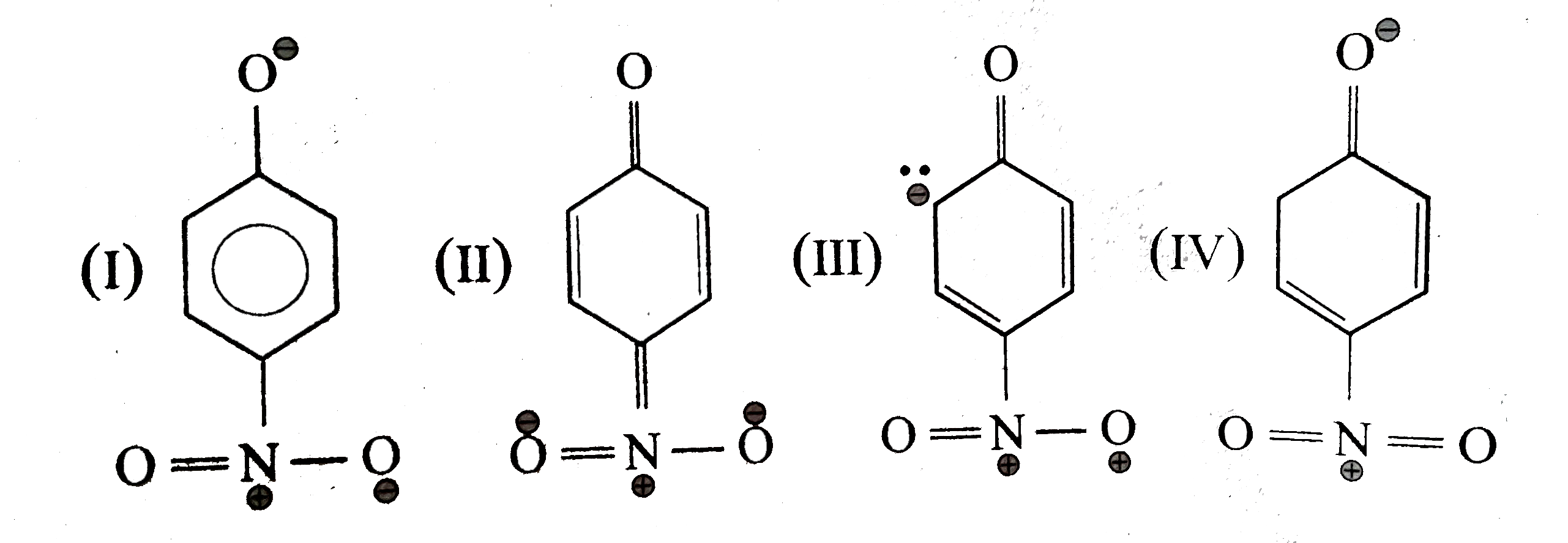 .
.
(f) Which of the more stable carbocation ?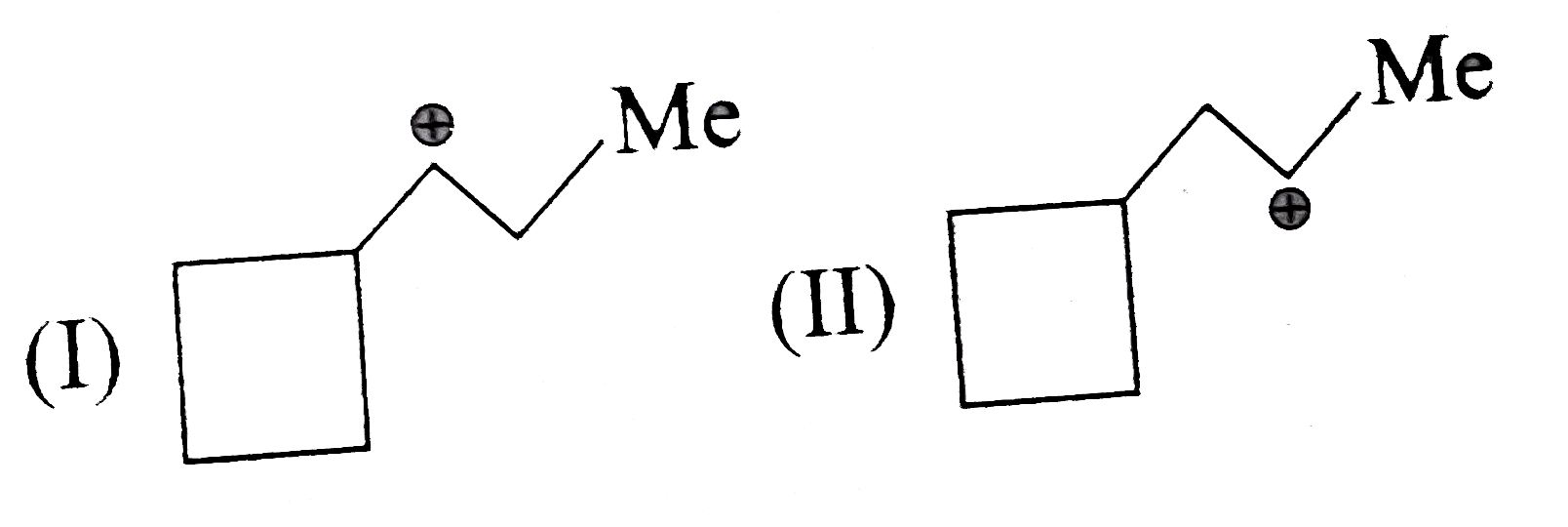 .
.
View SolutionThe tropical rainforest has a large population of animals. Explain why it is so.
View SolutionAccount for the following:
Although Fluorine has less negative electron gain enthalpy yet F2 is strong oxidizing agent.
View SolutionWhy N2 is more stable than O2 ? Explain on the basis of molecular orbital theory .
View SolutionManganese in the +2 oxidation state is more stable than +3 oxidation state where as iron is stable at +3 oxidation state than +2 oxidation state. Explain why
View Solution(a) (i) Write the disproportionation reaction of H3PO3
(ii) Draw the structure of XeF4
(b) Account for the following:
(i) Although Fluorine has less negative electron gain enthalpy yet F2 is strong oxidizing agent.
(ii) Acidic character decreases from N2O3 to Bi2O3, in group 15.
(c) Write a chemical reaction to test sulphur dioxide gas. Write chemical equation involved.
View SolutionExplain why (CH3)3C+ is more stable than CH3CH+2andCH+3 is the least stable cation.
View Solution

