Explain the following :
(a) Why MeNH⊕CH2 (I) is more stable more than MeCH2⊕CH2 (II) when both are 1∘ carbocations ?
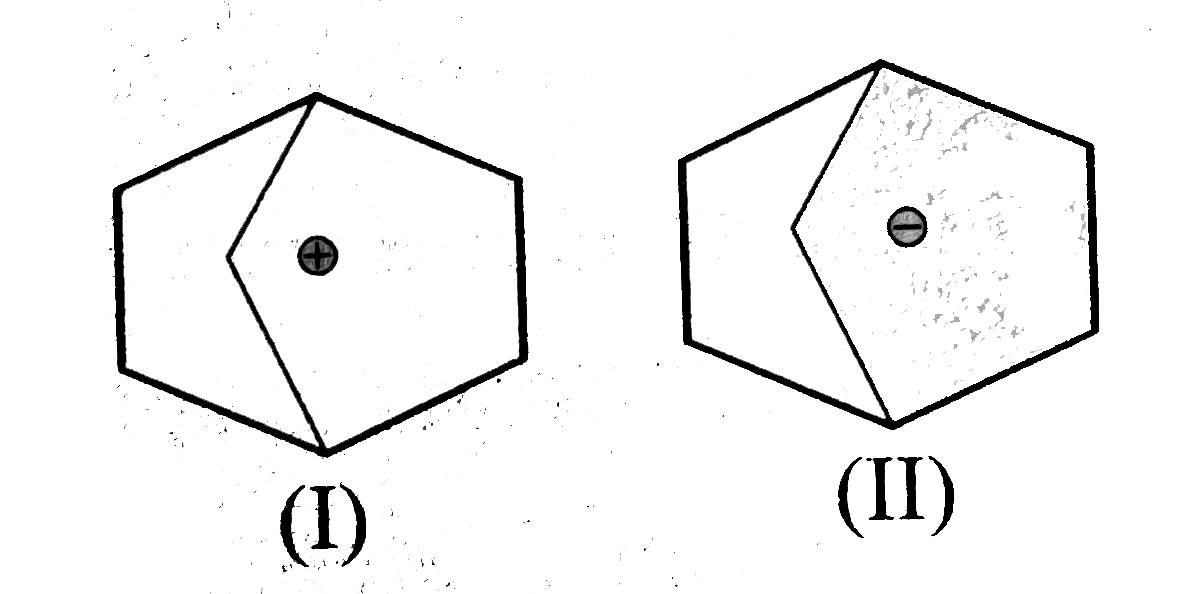
(b) Which of the following intermediate is unstable ?
( c) Why allylic free radical (CH2−CH=CH2)(I) is more stable than propy1 free radical (.CH2−CH2−CH3)(II), when both are 1∘ free radicals ?
(d) Arrange the following in the decreasing order of their stabilities :

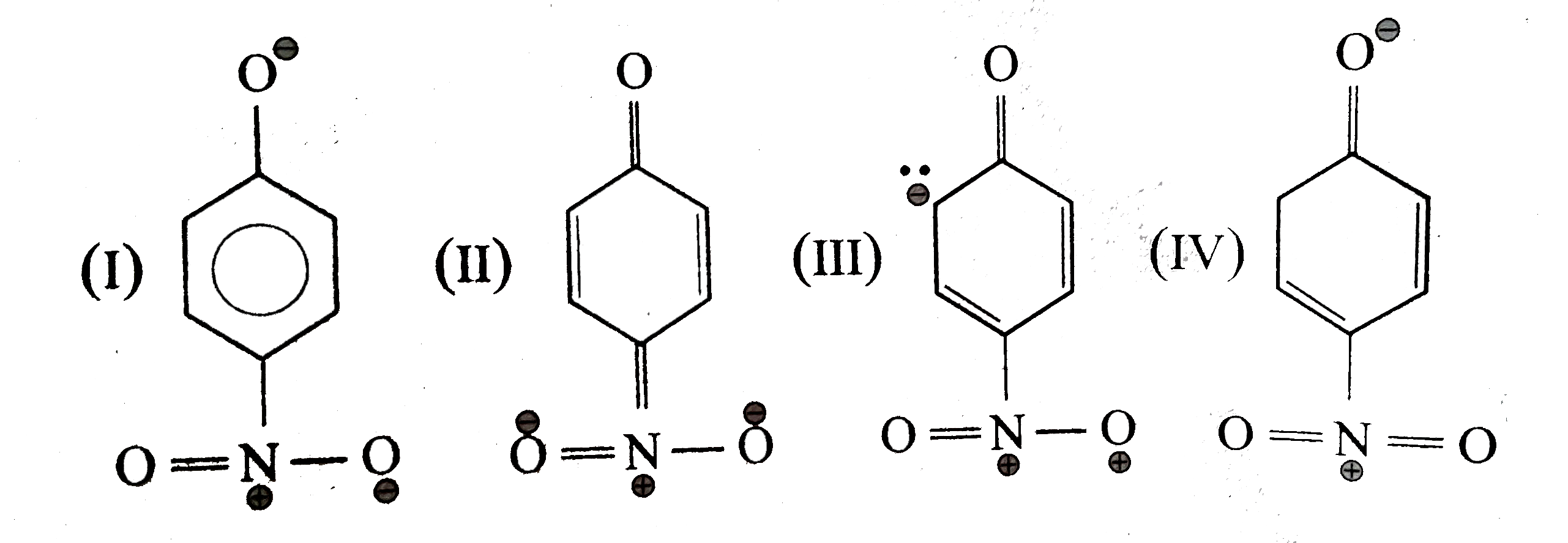
( e) Which of the following resonance structures of p−nitro phenoxide is not feasible ?
 .
.
(f) Which of the more stable carbocation ? 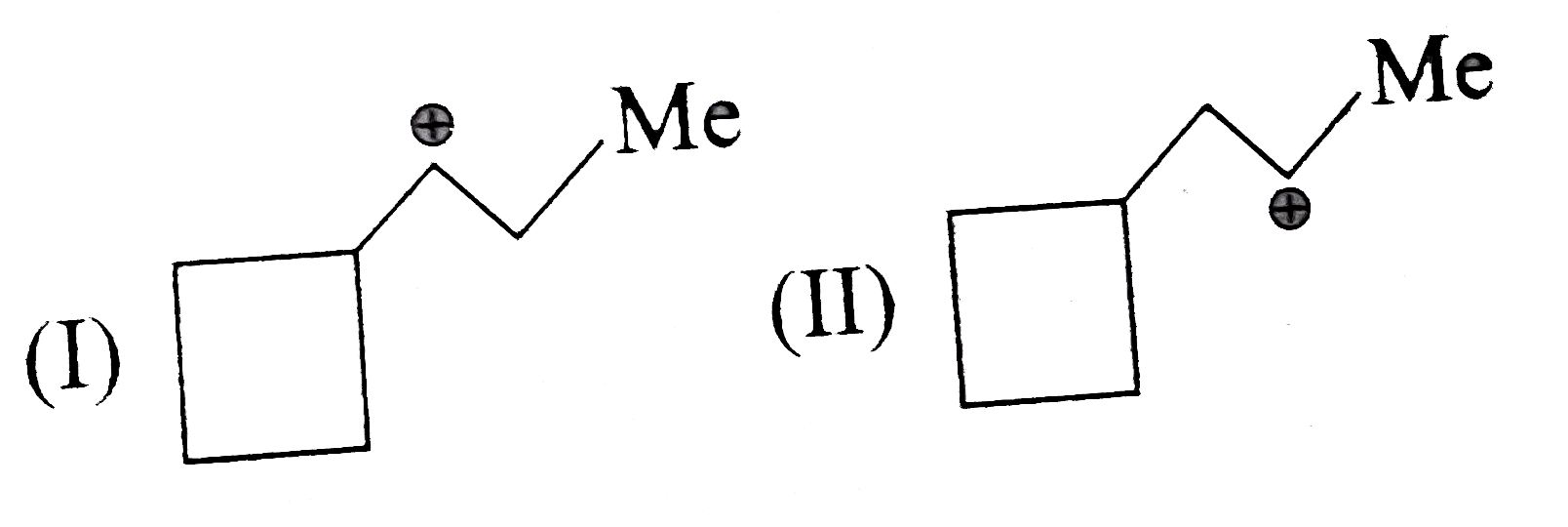 .
.
Video Solution
Text Solution
(a) In (I), positve charge on C atom is stabilised by resonance by the LP¯e,s on N atom.
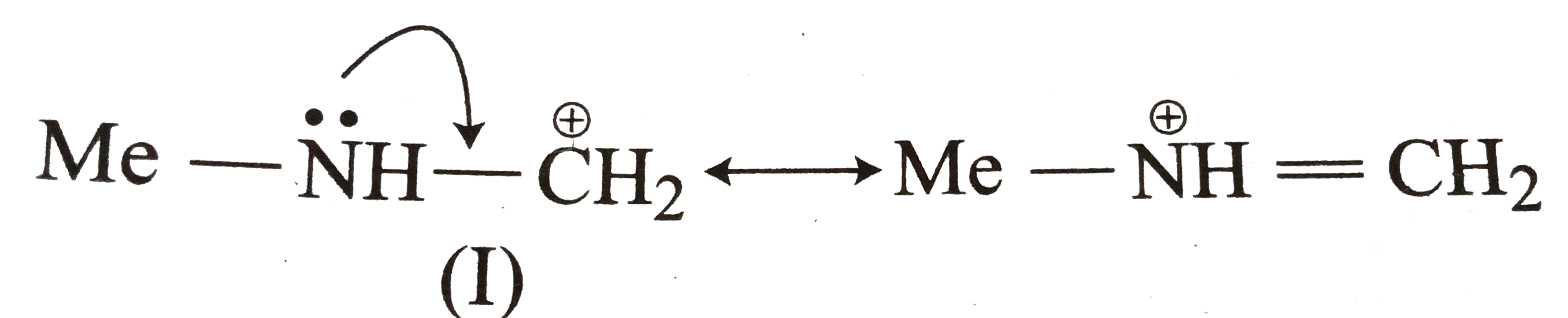
So, (I) is more stable than (II)
(b) (I) 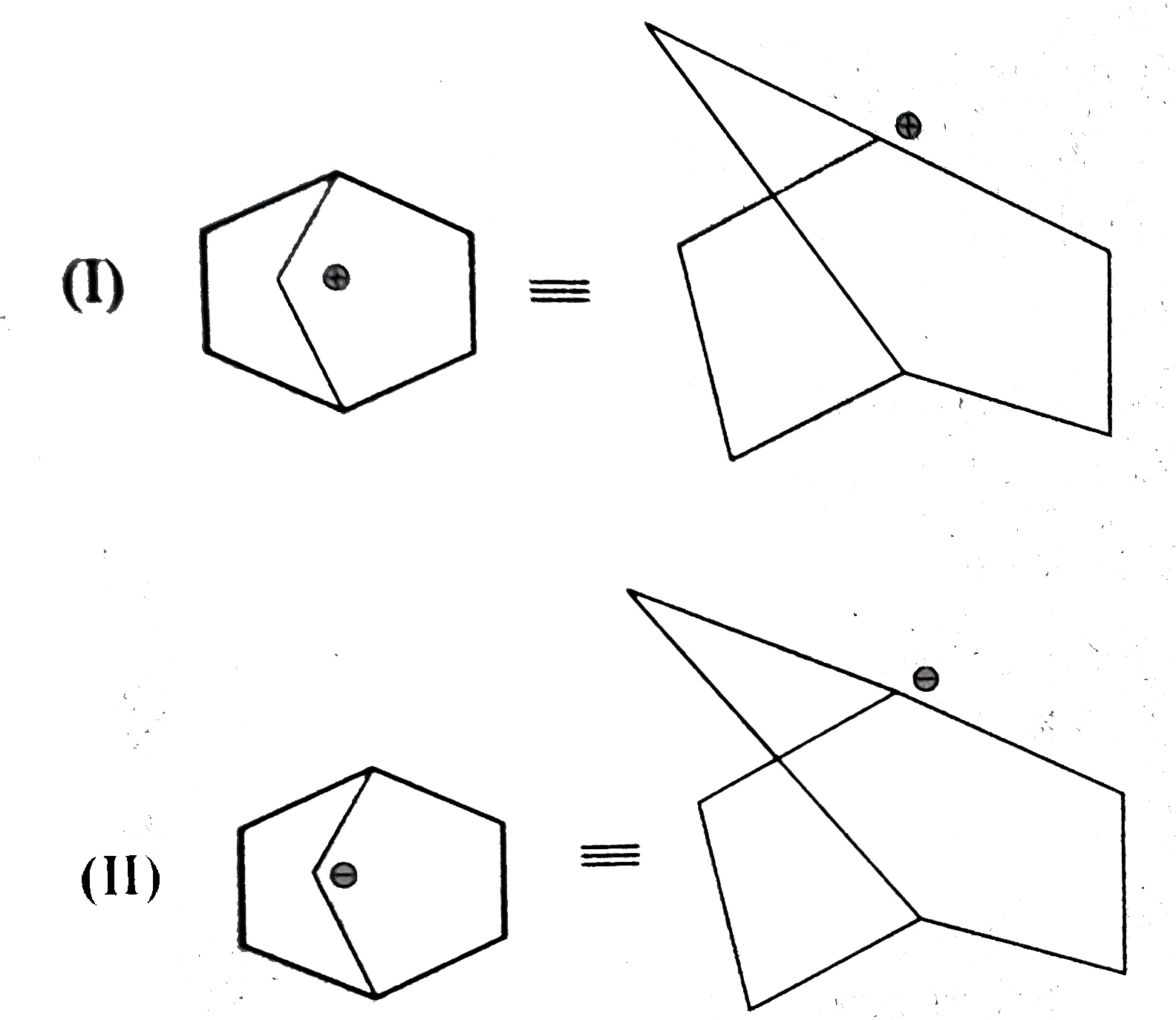
In (I), positive charge is present on bridgehead, so corbocation cannot attain planarity , hence (I) is less stable than (II).
( c) (I) is resonance stabilised, whereas (II) is not.
⋅CH2−CH=CH2↔CH2=CH−⋅CH2
≡⋅δCH2−−––––CH2−−––––⋅δCH2
(d) EDG stabilities carbocation, whereas EWG destabilises. ED power of : ..NH2(+R and −I)>−Me(+I and H.C effect), but +R>H.C>:.CH, triplet carbene have least tendency to release electron)>−NO2(−I and −R, net EW power).
So stability order is : IV>III>I>II
( e) (IV) is not feasible, because N is pentavalent with a positive charge.
(f) (II) is more stable since it has five α−H−atoms whereas (I) has three α−H−atoms. That is why (I) undergoes ring expansion by 1,2 - H^(Ө) shift. The shifts are always 1,2 not 1,3.

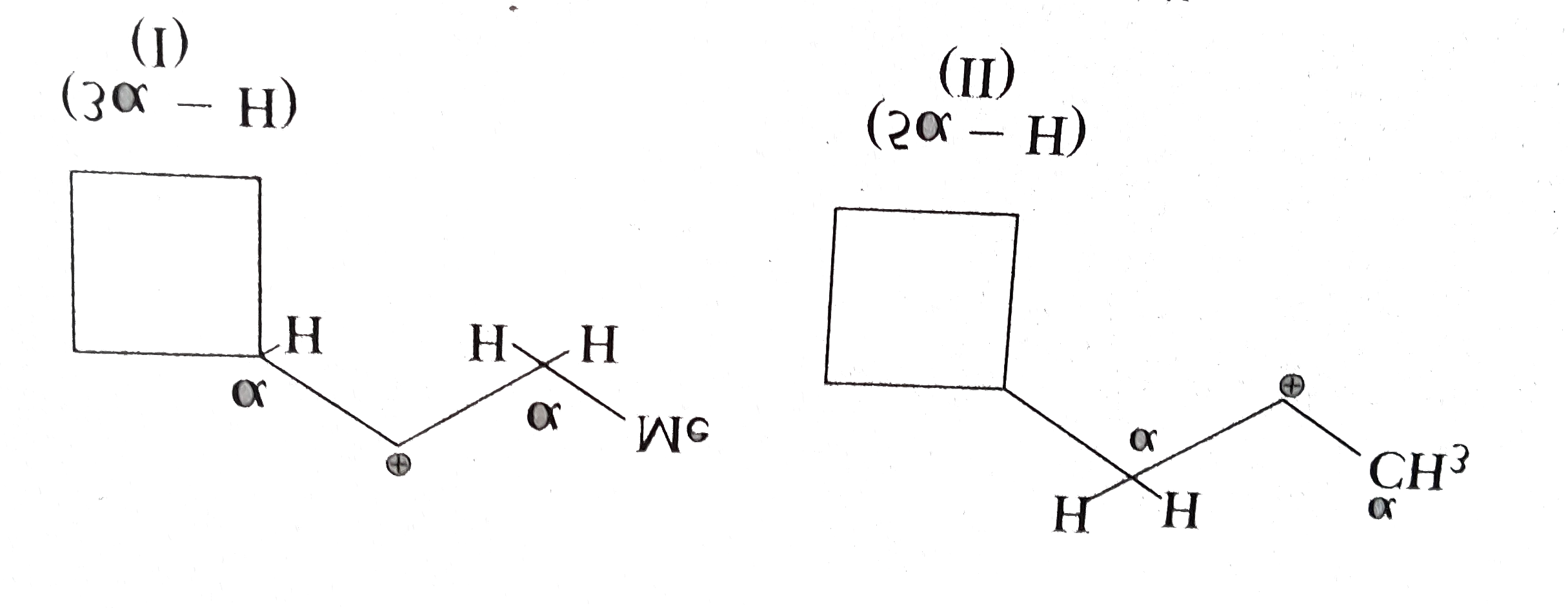
|
Topper's Solved these Questions
- GENERAL ORGANIC CHEMISTRYBook:CENGAGE CHEMISTRYChapter:GENERAL ORGANIC CHEMISTRYExercise:Subjective
Explore 11 Videos
- GENERAL ORGANIC CHEMISTRYBook:CENGAGE CHEMISTRYChapter:GENERAL ORGANIC CHEMISTRYExercise:Concept
Explore 16 Videos
- GENERAL ORGANIC CHEMISTRYBook:CENGAGE CHEMISTRYChapter:GENERAL ORGANIC CHEMISTRYExercise:Analytical and Descriptive
Explore 1 Video
- CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSBook:CENGAGE CHEMISTRYChapter:CLASSIFICATION AND NOMENCLATURE OF ORGANIC COMPOUNDSExercise:Analytical and Descriptive Type
Explore 3 Videos
- HYDROGEN, WATER AND HYDROGEN PEROXIDEBook:CENGAGE CHEMISTRYChapter:HYDROGEN, WATER AND HYDROGEN PEROXIDEExercise:Subjective Archive (Subjective)
Explore 3 Videos
Similar Questions
Knowledge Check
Identify the carbocations lesser stable than CH3−⊕CH2 :
A.⊕CH3 :BCH2=⊕CH(Vinyl Carbocation)C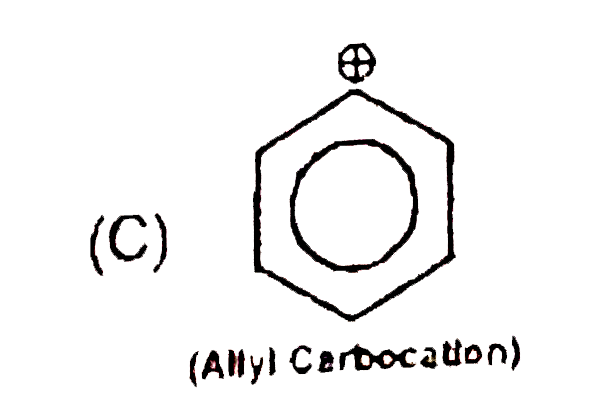 D
D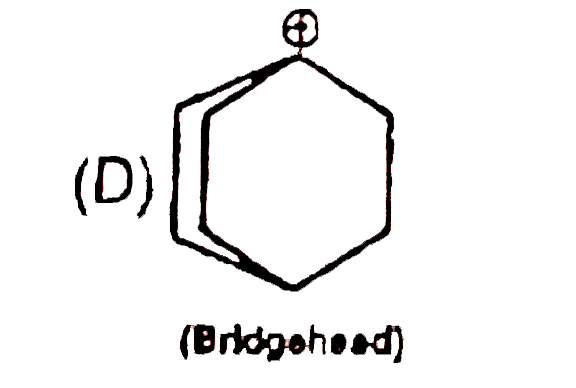
CH2=CH−CH=CH−CH3(I) is more stable than CH3−CH=C=CH−CH3(II) because
Athere is resonance is but not in IIBthere is tautomerism in I but not in IICthere is hyper conjugation in I but not in IIDII has more canonical structural than I.
Why is (CH3)3C+ more stable than CH3CH+2 ?
View SolutionExplain why (CH3)3+Cis more stable than CH3+CH2 and CH3 is the least stable cation
View SolutionExplain why (CH3)3+Cis more stable than CH3+CH2 and CH3 is the least stable cation
View SolutionExplain why (CH3)3C+ is more stable than CH3CH+2andCH+3 is the least stable cation.
View SolutionConsider the following free radicals.
(I)CH3−CH∘2
(II)Ph−CH∘2
(III)Ph−CH∘−CH3
The decreasing order of stability of the given free radicals is
View SolutionWhich of the O2NCH2CH2O− or CH3CH2O− is expected to be more stable and why ?
View Solution
