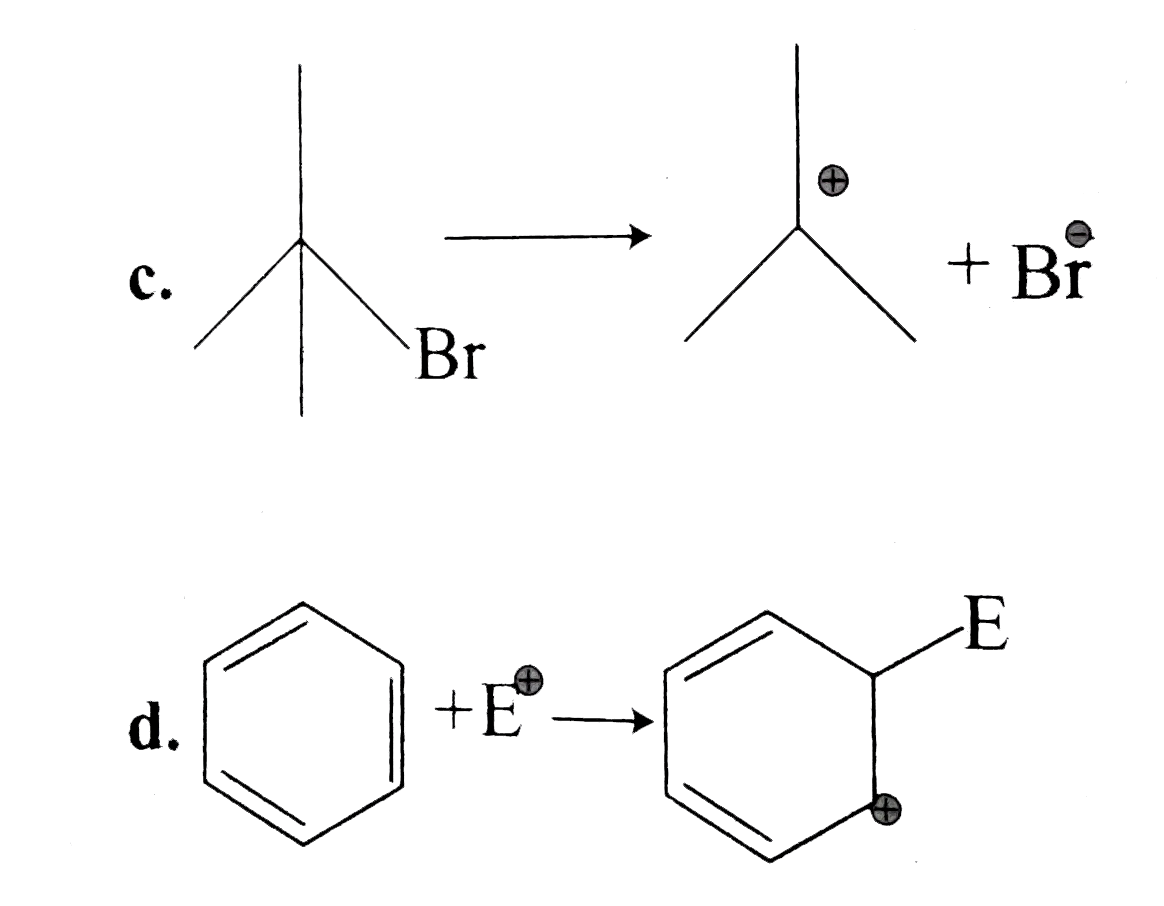
For the following bond cleavages use curved- arrows to show the electron flow and classify each as homolytic or hetrolytic fission.Identify reactive intermediate produced as free radical, carbocation and carbanion?
(a) 
(a)
Topper's Solved these Questions
Similar Questions
Explain about Kossel-Lewis approach to chemical bonding.
View SolutionFor the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion. (a) CH_(3)O-OCH_(3)rarrCH_(3)overset(.)O+overset(.)(O)CH_(3) (b)
View SolutionHomolytic fission of a covalent bond leads to the formation of
(a) electrophile
(b) nucleophile
(c) free radical
(d) carbocation
(e) carbanion
View SolutionFor the following bond cleavages, use curved-arrow to show the electron flow and classify each as homolysis or heterolysis. Identify intermediate products as free radical, carbocation, and carbanion.
(a) CH3O−OCH3→CH3.O+.OCH3
 .
.
View SolutionWrite about bond cleavage and intermediate free radical
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion (a) CH_(3)O- OCH_(3) rarr CH_(3) overset(.)(O) + overset(.)(O)CH_(3)
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion
(a) CH3O−OCH3→CH3.O+.OCH3
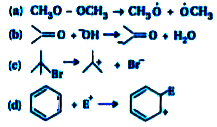
View SolutionFor the following bond cleavages, use curved arrows to show the electron flow and classify each as homolysis or heterolysis. Identify the reactive intermediates produced in CH_3-O-O-CH_3 rarr CH_3-O+CH_3O
View Solution

