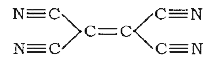How many σandπ bond are there in propylcyanide?
A
10σ,2π
B
8σ,2π
C
10σ,3π
D
11σ,2π
Text Solution
The correct Answer is:D
Propyl cyanide: H−H∣C∣H−H∣C∣H−H∣C∣H−C≡N
|
Similar Questions
Knowledge Check
How many sigma and pi bonds are there in tetracyano ethylene molecule?
A9σ and 9πB9σ and 7πC5σ and 9πD5σ and 7πHow many σandπ bonds are there in calcium cyanamide Ca(CN)2 ?
A2σand1πB2πand2σC1σand2πD2σand3π.How many σ−andπ−bond are there in salicycle acid?
A10σ,4πB16σ,4πC18σ,2σD16σ,2π
How many sigma and pi bonds are there in the molecule of tetracyano ethylene ? .
View SolutionCan a π− bond be formed without the formation of a σ− bond. How many σ−andπ− bonds are formed in acetylene?
View SolutionHow many σ and π bonds are there in the molecule C2(CN)4?
View SolutionHow many sigma and pi bonds are there in the molecule of tetracyano ethylene ? .
View SolutionHow many σandπ bonds are there in the molecule of tetracyanoethene ?
(NC)2C=C(CN)2
View Solution