Possible number of isomers of [Pt(Cl)(NO2)(NO3)(SCN)]2−, given it is a square planar complex, is _______.
Text Solution
The correct Answer is:12
In the complex Pt(Cl)(NO2)(NO3)(SCN)]2−, assume:
M=Pt a=Cl− b=NO−2 c=NO−3 d=SCN−
Now, it is (Mabcd] type complex, which exhibits three geometrical isomers as following:
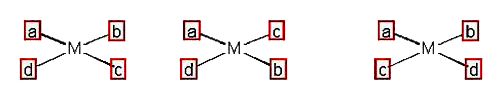
Now, NO−2SCN− are ambidentate ligands, hence, linkage isomerism is possible in each set of the three isomers.
The possible linkage combinations in each set of the three isomers are:
1.NO−2,SCN−
2.ONO−,SCN−
3.NO−2,NCS−
4.ONO−,NCS−
Total number of isomers=3×4=12.
|
Topper's Solved these Questions
Similar Questions
Knowledge Check
The total number of possible isomers of sqaure-planar [Pt(Cl)(NO2)(NO3)(SCN)]2− is :
A8B12C16D24Theortically possible number of isomers for [Pt(py)(NH3)(NO2)(Cl)(Br)(I)] are
A3B4C8D15Total number of possible isomers of complex [Pd(NH3)2(SCN)2]
A2B4C3D6
Give IUPAC name of linkage isomer of [(NH3)Pt(NO2)]Cl.
View SolutionThe total number of isomer shown by [Co(NH3)4(NO2)2](NO3) complex is :
View SolutionHow many geometrical isomers arepossible for [Pt(NO2)(NH2OH)(Py)]+?
View SolutionHow many total possible isomers are present for [Pt(CN)2(NO2)2]2− ?
View SolutionHow many total possible isomers are present for [Pt(CN)2(NO2)2]2− ?
View Solution

