The addition of HBr of 1-butene gives a mixture of products A,B and C
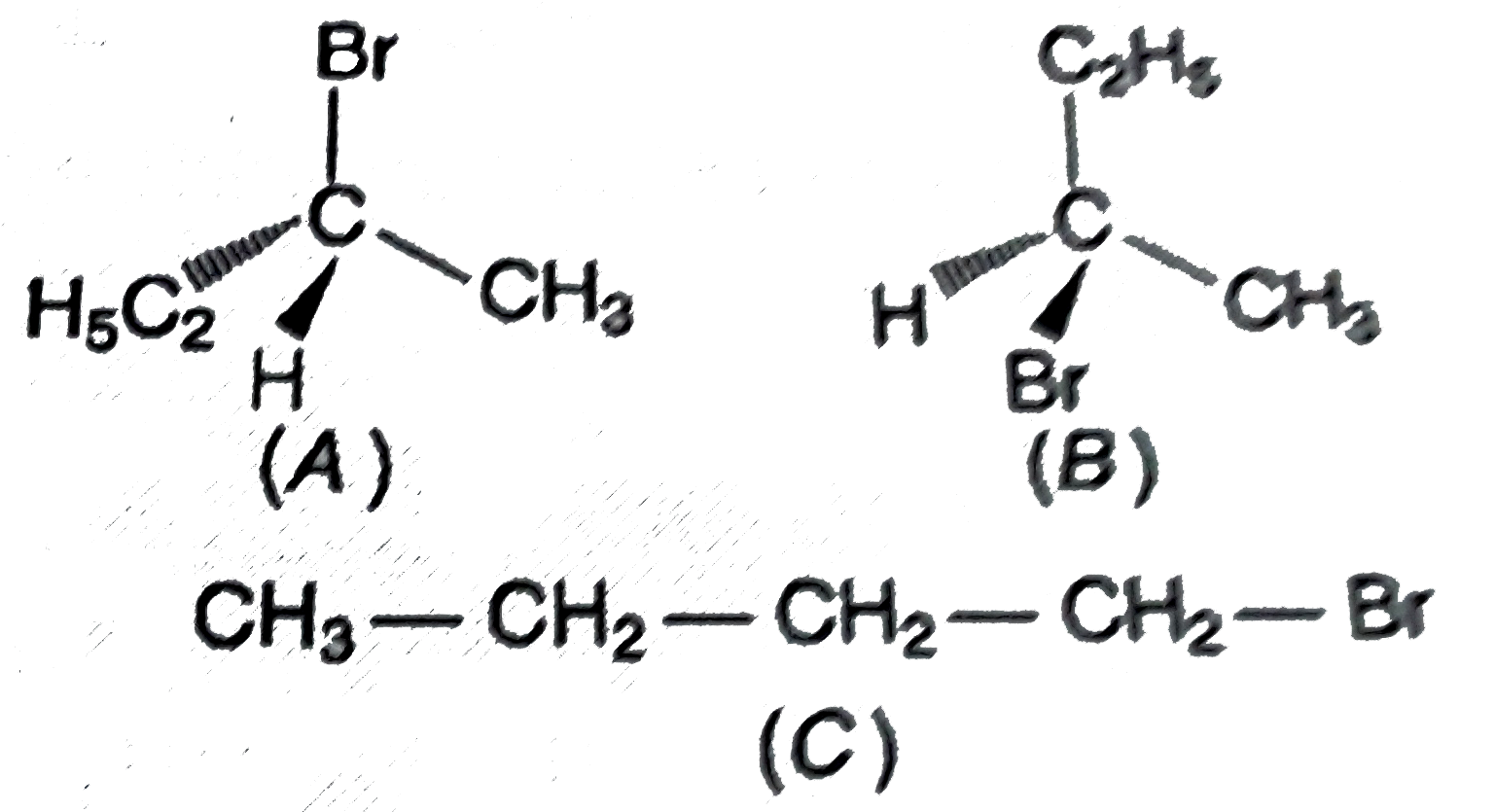
(C) CH3−CH2−CH2−CH2−Br
The mixture consists of
A and B as major and C as minor products
B as major, A and C as minor products
B as minor, A and C as major products
A and B as minor and C as major products
The correct Answer is:A
HBr selectively adds to the alkenes through Markownikoff's addition.
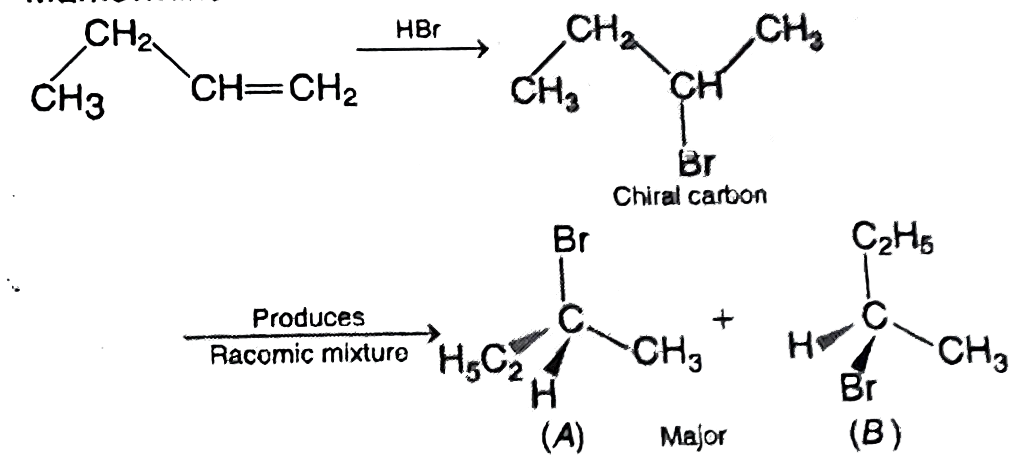
Alongwith A and B very little amounts of CH3CH2CH2C∣BrH2 is also produced in the reaction.
Similar Questions
Knowledge Check
The additio of HBr to but-1-ene gives a mixture of products (I),(II) and (III).
 .
.
(III). CH3−CH2−CH2−CH2−Br.
Mixture consists ofA(I) and (II) as major and (III) as minor productsB(II) as major, (I) and (III) as minor products.C(II) as minor, (I) and (III) as major products.D(I) and (II) as minor and (III) as major products.Br−CH2(CH2)2−Ch2−Br+CH3NH2→ product to the reaction is :
A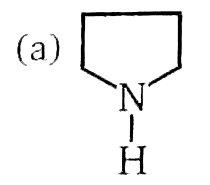 B
B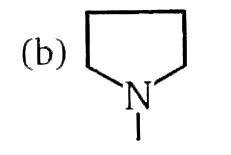 C
C D
D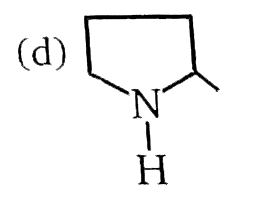
In the reaction
(CH3)2C−CH=CH2+HBr→
the chief product isA(CH3)2C∣Br−CH(CH3)2B(CH3)3C−CH∣Br−CH3C(CH3)3CH2CH2BrD(CH3)2CHC∣CH3HBr
Complete the following Reaction
CH3−CH=CH2+HBr
CCl3−CH=CH2+HBr
View SolutionGive the major product for the reaction
CH3−CH2−C∣OHH−CH3HBr−−→
View SolutionDefine racemic mixture.
Give IUPAC name of CH3−CH2−CH3∣CH−CHO
View SolutionSelect the alkyl halide and nucleophile that will give each of the following products.
(a) CH3−CH2−CH2−NH2
(b) CH3−CH2−O−CH2−CH3
(c) CH≡C−CH2−CH2−CH2
(d) (CH3)2CHS(CH3)2
(e) C6H5−O−CH2−CH3
View SolutionExplain the following:
i) Beta-1,3-diene gives 1,2- and 1,4-addition products.
ii) Addition of HBr to H2C=CHCH2C≡CH gives CH3CHBrCH2C≡CH.
iii) Alkenes in decreasing order of reactivity towards electrophilic addition.
a) CICH2CH=CH2
b) (CH3)2C=CH2
c) CH3CH=CH2
d) H2C=CHCl ltbgt iv) Stereochemical structure of the reaction product of Br2 with a) cis-2-butene and b) trans-2-butene.
v) Why alkynes are generally less reactive than alkenes shorter than that of n-butane.
vi) The central carbon-carbon bond in buta-1, 3-diene is shorter than that of n-butane.
vii) Arrange following alkenes in decreasing order of stability towards acid-catalysed hydrocarbons.
a) 1-phyenyl-1-butene, 1-phenyl-2-phenyl-2-butene
b) 2-methylpropene, cis-2-butene, trans-2-butene
c) 1-hexene, 2-methyl-1-pentene, 2-hexene.
View Solution