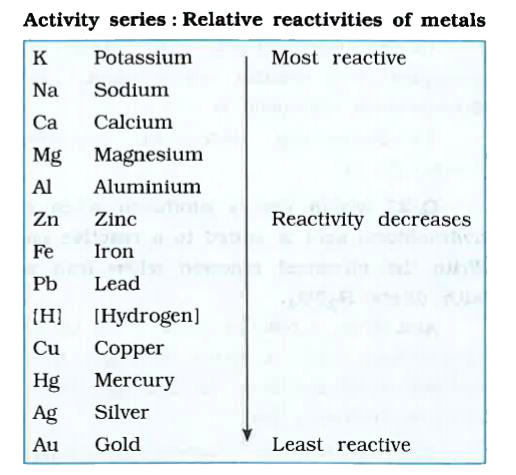
What is meant by reactivity or activity series of metals ? Arrange different metals in the decreasing order of their reactivity.
When different metals are arranged in decreasing order of their reactivity (or activity) then it constitutes a series called reactivity (or activity) series of metals. Reactivity (or activity) series for different metals is given in the following Table :
Topper's Solved these Questions
- METALS AND NON-METALSBOOK - KUMAR PRAKASHANCHAPTER - METALS AND NON-METALSEXERCISE - QUESTIONS AND ANSWERS (ACTIVITY)
53 Videos
- METALS AND NON-METALSBOOK - KUMAR PRAKASHANCHAPTER - METALS AND NON-METALSEXERCISE - TEXTUAL EXERCISE
19 Videos
- CHEMICAL REACTIONS AND EQUATIONS BOOK - KUMAR PRAKASHANCHAPTER - CHEMICAL REACTIONS AND EQUATIONS EXERCISE - PRACTICAL SKILL BASED QUESTIONS WITH ANSWERS
5 Videos
- MODEL QUESTION PAPER 01BOOK - KUMAR PRAKASHANCHAPTER - MODEL QUESTION PAPER 01EXERCISE - (SECTION-A) (MCQs)
1 Video
Similar Questions
(a). What is meant by the reactivity series of metals ? Arrange the following metals in an increasing order of their reactivities towards water:
Zinc, Iron, Magnesium, Sodium.
(b). Hydrogen is not a metal but still it has been assigned a place in the reactivity series of metals. Why?
(c). Name one metal more reactive and another less reactive than hydrogen.
(d). Name one metal which displaces copper from copper sulphate solution and one which does not.
(e). Name one metal which displaces silver from silver nitrate solution and one which does not.
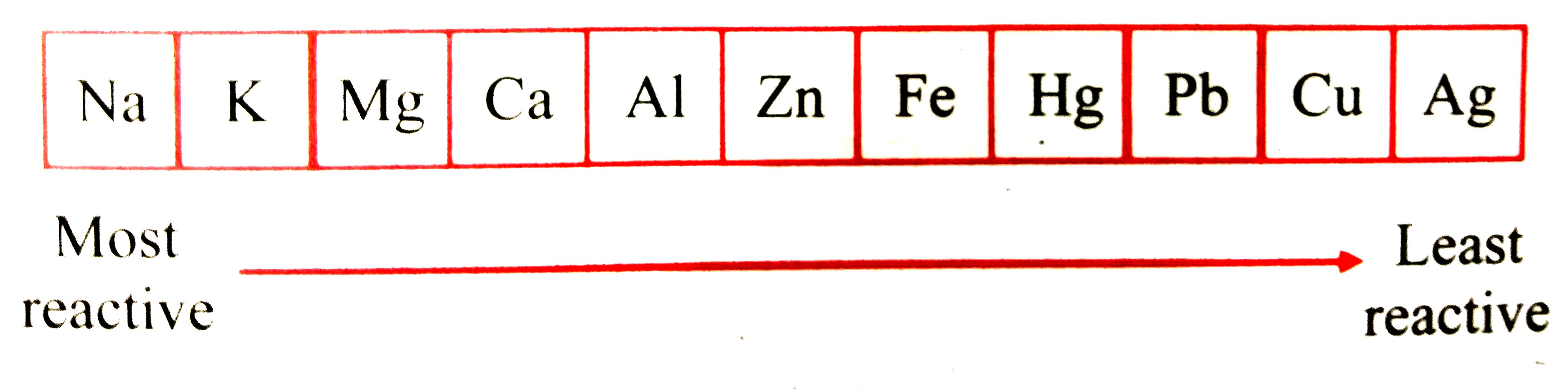
View SolutionIn the given reactivity series, some metals are misplaced. Rearrange these metals in the decreasing order of their reactivity.
View Solution
Knowledge Check
Arrange the following metals in the decreasing order of reactivity:
AIron > sodium > silver > goldBSodium > silver > iron > goldCGold > silver > iron > sodiumDSodium > iron > silver > goldArrange the following metals in the decreasing order of reactivity:
ASodium > silver > iron > goldBGold > silver > iron > sodiumCIron > sodium > silver > goldDSodium > iron > silver > goldArrange the followign metals in the decreasing order to reactivity Na, K, Cu and Ag.
ANa>K>C>AgBK>Cu>Na>AgCAg>Cu>Na>KDK>Na>Cu>Ag
Arrange the following metals in decreasing order of their reactivity.
Fe,Zn,Na,Cu,Ag
View SolutionWhat is meant by reactivity series of metal?
View SolutionWhat is meant by activity series of metals ?
View SolutionExplain the reactivity series of metals.
View SolutionArrange the metals Ca, Al, Mg and Zn in decreasing order of their reactivities :
View Solution
